Nome Completo: Ana Valéria Colnaghi Simionato
advertisement

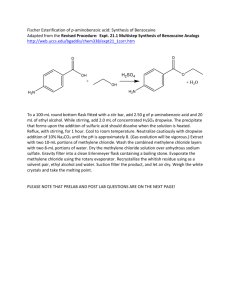
Sociedade Brasileira de Espectrometria de Massas – BrMASS Produtos Naturais e Sintéticos Synthesis and ESI(+)-MS/MS Study of N-alkyl-lactamic Aromatic Aminoesters Renato Sonchini Gonçalves1, Patrícia Verardi Abdelnur2, Rosineide C. Simas2 Marcos N. Eberlin2, Eduardo R. Pérez González1 eperez@fct.unesp.br Departamento de Física, Química e Biologia, Faculdade de Ciencias e Tecnologia, UNESP, C.P. 467, Presidente Prudente, 19060-900, SP, Brasil. 2 ThoMSon Mass Spectrometry Laboratory, Institute of Chemistry, State University of Campinas, Campinas, São Paulo, Brazil. 1 Compounds structurally related to p-aminobenzoic acid (PABA) have shown interesting pharmacological properties. PABA derivatives such as procaine, benzocaine and lidocaine are known as local anesthetic. [1] In this work we synthesized benzocaine like-compounds 1-4 by a nucleophilic aromatic substitution reaction (SNAr) of 4-chloro3-nitrobenzoic acid with N-(3-aminopropyl)-2-azepanone (APA) and N-(3-aminopropyl)2-pyrrolidone (APP) subsequently by coupled with acid catalyzed esterification using several aminoalcohols. Molecules containing the APA moiety have been reported as inhibitors of the human tryptase, an important mediator in the asthma pathology, while APP derivatives were found to be active against H2N2 and H3N2 strains of influenza virus. On the other hand, APP containing compounds have also been reported as HIV-1 protease inhibitors. [2] All the synthesized products have been characterized by NMR, FT-IR and Mass Spectrometry. It was realized a more detailed MS study of the products involving experiments using ESI-MS with high resolution mass to verify if the generated products had been the proposed chemical formula. To prove the structure of compounds here described we have performed MS/MS experiments using Argon as collision gas and the fragmentations confirmed the proposed structures. With these results, the products had been characterized and their structures were proving by ESI(+)-MS and ESI(+)-MS/MS. R O C O N R yield 56-70 % NO2 n( ) N NH 1. n = 1, R = Me 2. n = 3, R = Me 3. n = 1, R = Et 4. n = 3, R = Et O Figure. Synthesis of benzocaine analogues derivative by SNAr – Fisher esterification coupled reactions. Reference [1] Bhananker, S.M.; Azavedo, L.F.; Splinter, W.M. Pediat. Anesthes. 2008, 18, 140-144. [2] Ghosh, A.K., Leshchenko-Yashchuk, S., Anderson, D.D., Baldridge, A., Noetzel, M., Miller, H.B., Tie, Y., Wang, Yuan-Fang, Koh, Y., Weber, I.T., Mitsuya|, H. J. Med. Chem. 2009, 52, 3902–3914. 3º Congresso BrMass – 12 a 15 de Dezembro de 2009