Esterification exp
advertisement

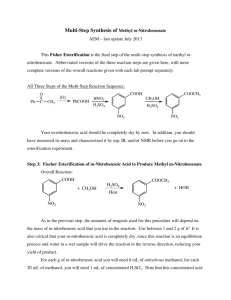
FISCHER ESTERIFICATION OF BENZOIC ACID (03/11/02) Carboxylic acids can be converted into esters by a number of methods. The most commonly used of these esterification methods is the acid-catalyzed reaction between carboxylic acids and alcohols. This is a reversible reaction with an equilibrium constant, Keq, approximately equal to 4 for most carboxylic acid - alcohol pairs. Thus when a carboxylic acid and an alcohol react in a 1:1 molar ratio, the ester is formed in ~67% yield. The yield of the ester can be increased by using a large excess of the alcohol to shift the equilibrium to the right (Le Chatelier's principle). A four-fold molar excess of the alcohol will produce the ester in ~95% yield. RCO 2H + R'OH H RCO 2R' H2O + Esterification using a large excess of an alcohol is known as the Fischer esterification procedure. Although esters may be formed in high yield (>95%) by Fischer esterifications, the esters are often isolated in appreciably lower yields (~75%). These lower isolated yields of esters are the result of the aqueous work-up normally used with Fischer esterifications to remove the excess alcohol and the acid catalyst. The aqueous alcohol solution formed when the reaction mixture is poured into water will dissolve an appreciable amount of the ester. In this experiment methyl benzoate will be prepared by a Fischer esterification of benzoic acid with methanol. CO2H + CH3OH H2SO4 CO2CH3 + H2O The methyl benzoate will be used in the subsequent experiment for the synthesis of a tertiary alcohol via a Grignard reaction. Procedure: Place 8.00g (0.0656mol) of benzoic acid and 25mL (0.617mol) of methanol in a 100mL roundbottomed flask, and carefully pour 3.0mL of concentrated sulfuric acid down the walls of the flask. Swirl to mix the reactants, add 2-3 boiling stones, attach a condenser, and heat the mixture at reflux with a thermowell for 30 minutes. Cool the reaction mixture and pour it into 75mL of water contained in a separatory funnel, taking care to keep the boiling stone from entering the separatory funnel. Rinse the reaction flask with 35mL of ether, pouring it into the separatory funnel. Stopper, shake, and drain off the aqueous layer which contains the sulfuric acid and most of the methanol. Wash the ether layer with another 15mL of water, drain, and then wash with two 25mL portions of 5% sodium bicarbonate. Cautiously shake, venting often to release pressure, until no further reaction is apparent. Wash with saturated sodium chloride solution (15-20mL) and dry the ether layer with anhydrous sodium sulfate. Decant the ether layer into an appropriate size, tared round-bottomed flask (solution should no more than half fill the flask) and evaporate the ether on a rotary evaporator. Calculate a percent yield. Dissolve some of your product in chloroform as directed by your instructor. Fill an IR solution cell with this solution and obtain an IR spectrum. Save the methyl benzoate in a tightly stoppered container for use in a Grignard synthesis scheduled for the next lab period. Note: Do not use your 100mL round-bottomed flask for this purpose because it will be needed for the preparation of the Grignard reagent during the next lab period. % Concentrated sulfuric acid is very corrosive! % Keep ether contained or in a hood at all times! % Vent the separatory funnel often after the addition of sodium bicarbonate: CO2(g) is generated from the reaction of H2SO4 + NaHCO3! % None of the reagents in this experiment need to be discarded in the halogenated waste. The water-soluble/aqueous reagents may be discarded in the sink w/ running water. · To save time, obtain the solutions and set up for the washing steps while the reaction mixture undergoes reflux. POSTLAB EXERCISE: FISCHER ESTERIFICATION OF BENZOIC ACID (03/11/02) Ø Due in the lab following the completion of the experiment (usually one week). No papers accepted after the Friday of the lab week it’s due (absence or no absence!). Please answer questions on this form. (30pts TOTAL) 1. PRODUCT INFORMATION (5pts) %Yield (5pts): 2. (12pts) Assuming Keq = 4.00 for the esterification of benzoic acid: (a) (6pts) What is the maximum percent yield of methyl benzoate that would be produced under the reaction conditions used in this experiment (b) (6pts) How many moles of methanol would have to be used to produce methyl benzoate in 99% yield? 3. (5pts) If one wanted to force the acid-catalyzed esterification toward completion without using a large excess of methanol, how might this be accomplished? 4. (8pts) Compare the IR spectrum of methyl benzoate with the IR spectrum of benzoic acid (http://www.chem.mtu.edu/kmroth/Organic/Manuals/Ester/Fischer.htm) Please make functional group assignments and comparisons directly on the spectrum.