Benzocaine Synthesis: Chemistry Pre-Lab Questions
advertisement

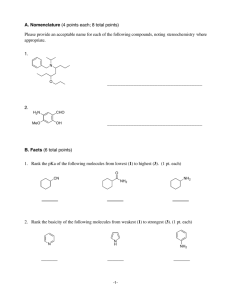
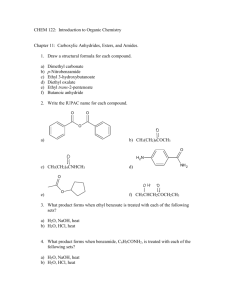
CH 421, Pre‐lab 5 Name___________________________ Spring 2011 Synthesis of Benzocaine (Pavia Exp. 44, 343‐350; refer to McMurry Chapter 21) O OH O OEt i. EtOH, H2SO4, reflux ii. Na2CO3, H2O NH2 para-aminobenzoic acid (PABA) NH2 ethyl 4-aminobenzoate (benzocaine) 1) The reaction is set up by first dissolving para‐aminobenzoic acid in absolute ethanol, then adding concentrated sulfuric acid. The procedure indicates a large amount of precipitate forms at this point. Draw the structure of this precipitate. 2) The Fischer esterification reaction is catalytic in acid. However, in this particular reaction, more than one equivalent of sulfuric acid is used. Briefly explain why. 3) At what temperature is this reaction mixture heated? 4) After refluxing for one hour, the reaction mixture is cooled to room temperature then poured into water. Sodium carbonate is then added slowly, as bubbling will occur. What gas is evolved? 5) Draw the structure of the precipitate formed when sodium carbonate is added to the aqueous workup mixture. 6) If methanol were used in this reaction instead of ethanol, draw the resulting product. 7) Based on the mechanism of the Fischer esterification reaction, indicate whether route A or route B would lead to the oxygen‐18 labeled ester product. 8) In the IR spectrum of benzocaine, two of the three peaks in the 1000‐1300 cm‐1 range are due to the C‐O bonds. What is the third peak in the 1000‐1300 cm‐1 range due to? Also, assign the C=O and the N‐H peaks below. 9) Assign the peaks in the 1H NMR spectrum of para‐aminobenzoic acid (90 MHz, d6‐DMSO). O OH d c H2N b a 1H 2H 2H, 2H 10) Assign the peaks in the 1H NMR spectrum of ethyl‐4‐aminobenzoate (90 MHz, CDCl3). O OCH2CH3 H2N a d c e b 2H 2H 2H, 2H 3H 11) Complete the following table and include it in your notebook prior to the lab. O OH O OEt i. EtOH, H2SO4, reflux ii. Na2CO3, H2O NH2 para-aminobenzoic acid (PABA) NH2 ethyl 4-aminobenzoate (benzocaine) Name formula MW (g/mol) mass 1.2 g mmol equiv Theor. mass = Theor. mmol = ____ _____ p‐amino‐ benzoic acid ethanol sulfuric acid ethyl‐4‐ amino‐ benzoate (benzo‐ caine) d (g/mL) vol _____ ___ mp (°C) bp (°C) ____ 12 mL ____ 1 mL ____ Lit. mp = ___ ___