click here
advertisement

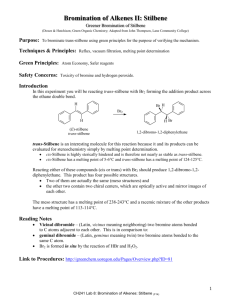
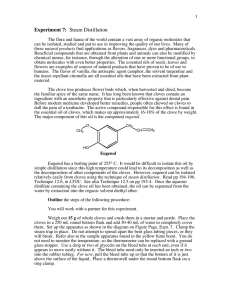
Courtney Courter Organic Chemistry (Night) October 7, 2010 Lab Experiment #5 Bromination of Stilbene Purpose of the Experiment: To make dihalides by brominating alkenes. Material: 1 gram of stilbene, 30 mL diethyl ether, addition 15 dimethyl ether (5 ml and than 10mL), 7 mL of 5% Bromine solution Equipment: 100 mL round bottom flask, Claisen adapter, 125 mL seperatory funnel, condenser, oven, stirring rod, Keck clip, buret, glass stopped, suction filtration equipment, buchner funnel, melting point Procedure: Dry the 100 mL round bottom flask, Claisen adapter, 125 mL seperatory funnel, and condenser in the oven for a half hour. Place 1 gram of stilbene into the 100 mL round bottom flask. Add 30 mL of diethyl ether and stir under hood. Use the dried claisen adapter and condensor (attached by the keck clip) to attach to the round bottom flask. Obtain 7 mL of 5% Bromine solution and put it into the 125 mL separatory funnel. Cover the funnel with a glass stopper. Connect the funnel to the claisen adapter. Transfer the bromine into the round bottom flask over a period of ten minutes. (dropwise) After completion stir for 15 more minutes until complete discoloration of bromine. Suction Filtrate out your solid. You may use extra 5 mL of ether to transfer the solid. Wash the solid on ur funnel with 10mL of ether to remove traces of bromine. Set the solid aside to dry for a week. Than determine weight and melting point. Table of Physical Constants: The 4 Possible Compounds Stilbene Molecular Weight 180.25 g/mol Melting Point 122-125 °C (E Stilbene) Bromine 136.2 g/mol Diethyl Ether -7.2 °C -116.3 °C 74.12 g/mol Meso stilbene dibromide 340 g/mol 236-240ºC Observations: As we continued to ass drops of bromine and stir the color became a light orange. The solid was a white-ish color. Data Collected: Weight of solid product Melting Point of solid Product Data 1.247 240 – 250 ºC Evaluation: Based on the melting points and weights we can determine that the two compounds of the mixture are most likely the E isomer of Meso stilbene dibromide Courtney Courter Organic Chemistry (Night) Final Report: a. Theoretical Yield: b. Actual Yield and percent: c. MP Range: d. Newman Projection: e. October 7, 2010