Document
advertisement

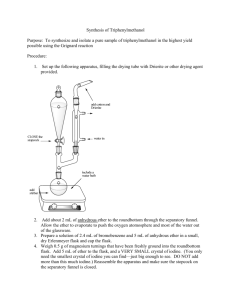
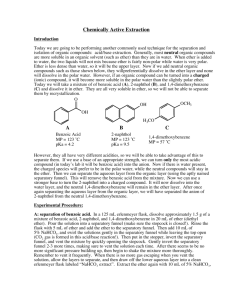
Britney Ongley Ashrit CHEM 3105-301 June 9, 2015 Separating an Acid from a Hydrocarbon Purpose: The purpose of this experiment is to extract a neutral carboxylic acid and its anionic conjugate base using the difference in their water solubility, from a neutral hydrocarbon. Reaction and Physical Properties Table: 1. Water-insoluble/Ether-soluble Benzoic acid is treated with water-soluble Sodium hydroxide to produce the water-soluble ionic conjugate base of Benzoic acid and water. 2. Water-soluble ionic conjugate base of Benzoic acid is treated with Hydrochloric acid to regenerate water-insoluble/Ether-soluble Benzoic acid and water-soluble Sodium chloride. Compound Naphthalene Biphenyl Benzoic acid oChlorobenzoic Acid Diethyl ether 2 M NaOH 6 N HCl sat’d NaCl MW 128.17 154.21 122.12 156.57 Amount 74.12 40 mL 40 mL 8 mL 10 mL mp (°C) 78-80 68-70 120-122 140-142 bp (°C) d 35 0.7184 Safety: Diethyl ether: Risk statement: Extremely flammable liquid and vapor, harmful if swallowed, causes serious eye irritation, may be harmful if inhaled, may cause drowsiness or dizziness. Safety statement: Keep away from heat/sparks/open flames/hot surfaces, avoid breathing dust/ fume/ gas/ mist/ vapours/ spray, if in eyes: rinse cautiously with water for several minutes. Remove contact lenses, if present and continue rinsing. Sodium hydroxide: Risk statement: Causes eye, skin, digestive, and respiratory tract burns. Hygroscopic (absorbs moisture from the air). Safety statement: In case of skin/eye contact, immediately flush eyes/rinse skin with plenty of water for at least 15 minutes. Get medical aid immediately. If swallowed, do NOT induce vomiting. Get medical aid immediately. If victim is fully conscious, give a cupful of water. Never give anything by mouth to an unconscious person. Procedure: **** Keep a record/flowchart of the procedure and make sure you label all your flasks. **** 1. Make sure all glassware is clean and labeled. 2. Put 40 mL of 2 M NaOH solution in a 150 mL Erlenmeyer flask and place the flask in an ice bath. 3. You will be given a 0.50 g sample of unknown composition; record the sample number and weigh it to three decimal places. 4. Set aside a few mg’s of the sample for the next experiment(just enough to see) and pour the rest into the separatory funnel. 5. Add approximately 30 mL ether and stir so the solid may dissolve. 6. Cautiously add NaOH by 5-10 mL increments until the aqueous layer of the solution becomes steadily basic. You can check the pH using red litmus paper. * The reaction will get hot due to the exothermic nature of acid-base reactions. Ether’s boiling point is very low so the solution may boil.* 7. Decant the aqueous layer into an Erlenmeyer flask labeled “basic extract”. 8. Wash the organic layer by adding 10 mL of distilled water to a separatory funnel, shaking, venting, and draining the water. 9. Transfer the ether layer into another Erlenmeyer flask labeled “ether wash”. 10. Return the basic extract to the funnel and wash it with 10 mL of fresh ether. Drain the aqueous layer into the basic extract flask. 11. Add the ether wash to the ether in the separatory funnel and add about 10 mL saturated aqueous NaCl to the ether wash as well. 12. Drain the water layer into the basic extract flask, and drain the ether into the ether wash flask. 13. To remove the traces of water left in the ether, add a small amount of your drying agent, magnesium sulfate, to the ether. 14. Add about 8 mL of 6 N HCl to recover the acid from the basic extract, and check the pH to see if it is from 1-2. If it is higher, add more HCl. 15. Collect the precipitate that should have formed using vacuum filtration, via a Hirsch funnel. 16. Wash the flask with 10-15 mL cold distilled water and save this sample for next lab period. Allow it to dry on a piece of filter paper in glassware drawer. 17. Pour the leftover aqueous phase down the drain because it contains only water, NaCl, and dilute HCl. 18. Pour the ether through a conical filter to separate it from the magnesium sulfate and evaporate the ether in the hood. Store the remaining hydrocarbon in a covered shell vial until next lab period. Observations: Three-fourths of the 0.50 g unknown sample will dissolve within three seconds after the addition of 2 M NaOH. The fourth that remained was made up of the bigger precipitates of the sample that has yet to dissolve and is collected at the bottom. After you have added more NaOH in increments and checked the acidity, the sample should be fully dissolved. Later in the lab, when adding the HCl, we added 10 additional mL of HCl solution in order to get the aqueous layer to remain acidic. After this addition, white precipitate crystals formed at the bottom of the flask. ********No calculations were needed for this experiment.********* Conclusion: The extraction of a neutral carboxylic acid and its anionic conjugate base from the neutral hydrocarbon in the ether solution was a success. The acidic and basic solutions were able to be separated due to the differences in their densities. Throughout the lab, the acidic layer was always on top and the basic layer predominately comprised the bottom of the funnel. Once the hydrocarbon was in the ether solution, we were able to isolate the carboxylic acid by drying it. We were able to save these crystals for the next lab where we will identify the sample. Post Lab Questions: 1. Water is able to hydrogen bond, while ether cannot. The intermolecular forces in water need more energy to break the bonds, making the boiling point of water higher than ether. 2. In order to separate the compounds, you must add them to ether before adding hydrochloric acid to make the compound (R-NH3+-Cl) water soluble. You need to convert the hydrocarbon in its organic phase to its basic version by adding sodium hydroxide. This will precipitate the amine, which needs to be filtered out, and you will use the hydrogen to evaporate the ether.