Electron Configuration Worksheet
advertisement
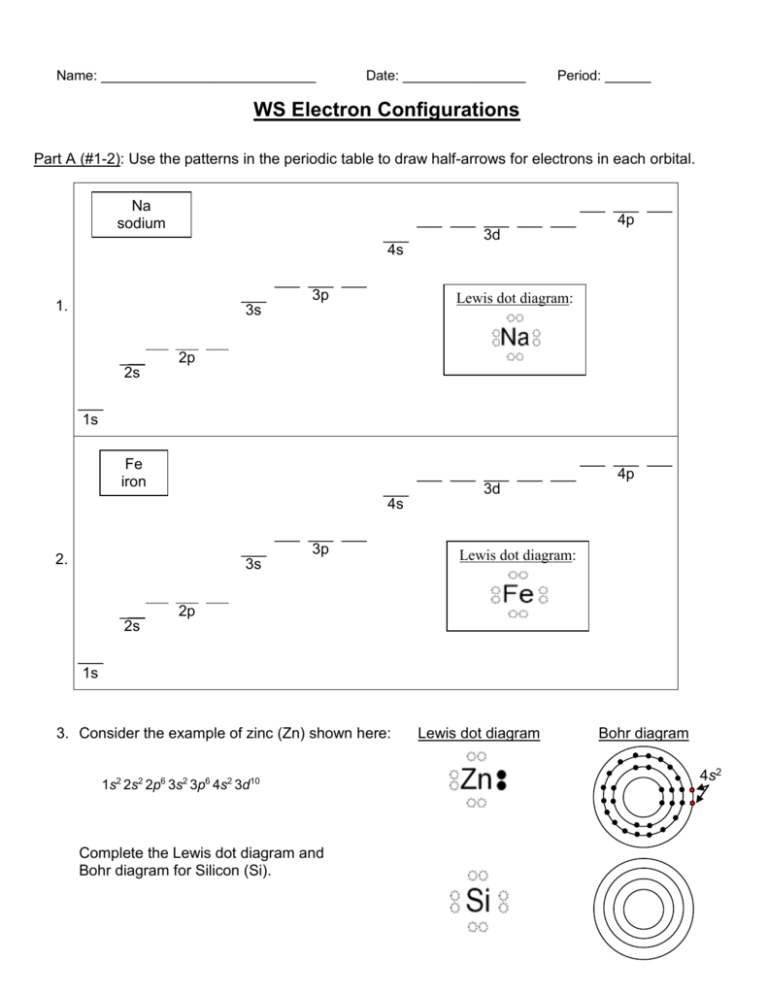
Name: ____________________________ Date: ________________ Period: ______ WS Electron Configurations Part A (#1-2): Use the patterns in the periodic table to draw half-arrows for electrons in each orbital. Na sodium ___ ___ ___ ___ ___ ___ 3d 4s ___ 3s 1. ___ 2s ___ ___ ___ 3p ___ ___ ___ 4p Lewis dot diagram: ___ ___ ___ 2p ___ 1s Fe iron ___ ___ ___ ___ ___ ___ 3d 4s ___ 3s 2. ___ 2s ___ ___ ___ 3p ___ ___ ___ 4p Lewis dot diagram: ___ ___ ___ 2p ___ 1s 3. Consider the example of zinc (Zn) shown here: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 Complete the Lewis dot diagram and Bohr diagram for Silicon (Si). Lewis dot diagram Bohr diagram 4s2 Part B (#4-20): Use the patterns in the periodic table to electron configurations for the following atoms. valence e– Electron Configuration Symbol Group # Total # (on highest level) # e– 4. Li 5. N 6. F 7. Ne 8. Na 9. Mg 10. Al 11. Cl 12. Ar 13. K 14. Ca 15. Br Part C (#16-20): Write the name of the element, number of valence electrons, and group number. 16) 1s22s22p1 ________________ # valence e– _____ Group # ____ 17) 1s22s22p4 ________________ # valence e– _____ Group # ____ 18) 1s22s22p63s23p2 ________________ # valence e– _____ Group # ____ 19) 1s22s22p63s23p64s23d2 ________________ # valence e– _____ Group # ____ 20) 1s22s22p63s23p64s23d104p65s1 ________________ # valence e– _____ Group # ____ Part D: Use the info from #1-20 to answer the following questions. 21) What do you notice about the group # and the # of valence e – for Li, Na, and K? ______________________________________________________________________ 22) What do you notice about the group # and the # of valence e– for Mg and Ca? ______________________________________________________________________ 23) What do you notice about the group # and the # of valence e – for F, Cl, and Br? ______________________________________________________________________ 24) What can you conclude about the group # and the number of valence e – ? ______________________________________________________________________











