What Element Am I
advertisement

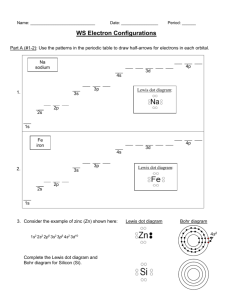
Name: ________________________ Period: ________ Date: ______________________ What Element Am I? Element #1 1. I am a gas at room temperature. 2. I have seven valence electrons. 3. I am in period 3. Element #2 1. I am brittle, but I conduct electricity. 2. I have a charge of – 3. 3. I have 51 protons in my nucleus. Noble-gas core e. configuration: Lewis valence electron dot diagram: Metal, nonmetal, or metalloid? Metal, nonmetal, or metalloid? Element #3 Element #4 1. I am lustrous, malleable and ductile. 2. My charges and oxidation numbers vary. 3. I have two more protons than iron. 1. I am silvery and conduct electricity. 2. I’m reactive, but not as reactive as the alkali metals. 3. My atomic number is 38. Noble-gas core orbital diagram: Noble-gas core e. configuration: What is my chemical family? Lewis valence dot diagram: Element #5 Element #6 1. I’m chemically unreactive. 2. I don’t get a negative or positive charge. 3. I’m in period 2. 1. I have six valence electrons. 2. I do not conduct heat or electricity. 3. I am a gas at room temperature. Lewis valence electron dot diagram: Ground state electron configuration: What is my chemical family? Metal, nonmetal, or metalloid? Name: ________________________ Period: ________ Date: ______________________ Element #7 1. My noble-gas core electron configuration is [Kr] 5s2 4d10 5p3. Element #8 1. I have a charge of +1. 2. I have the largest atomic mass in my group. Charge: Three properties: Lewis valence dot diagram: Metal, nonmetal, or metalloid? Lewis valence electron dot diagram: Element #9 Element #10 Element #11 Element #12 Name: ________________________ Period: ________ Date: ______________________