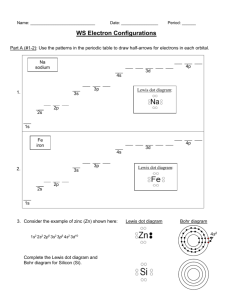
C:\office new\teaching\CHM 109 F 12\homework\Sci not Lewis v3.wpd
advertisement
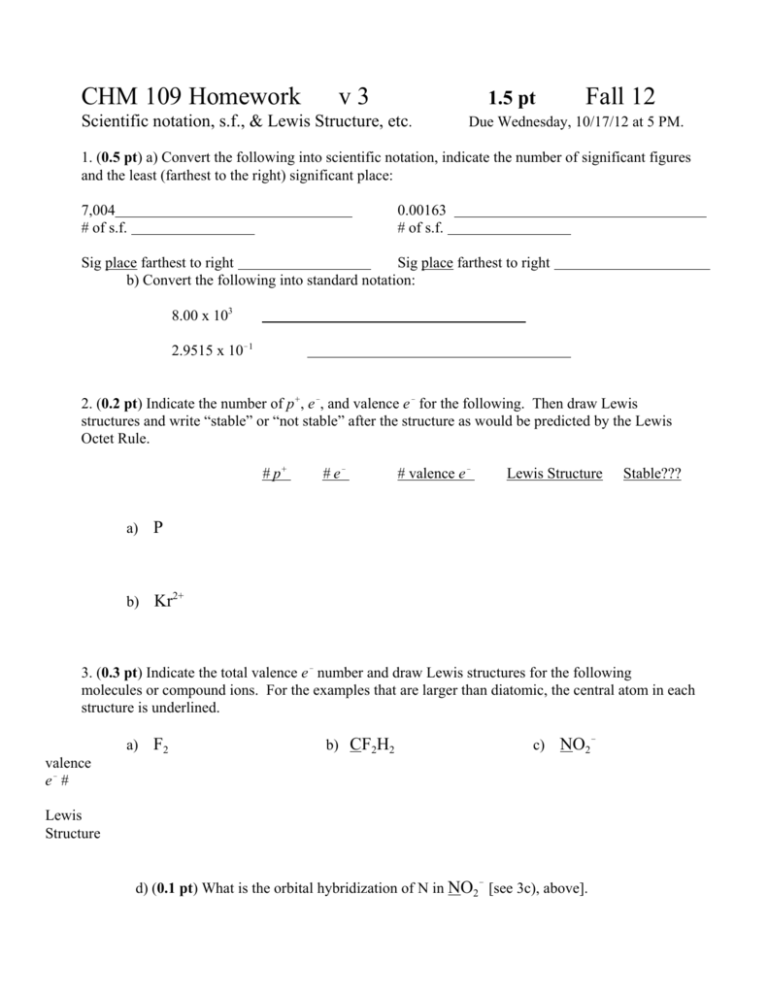
CHM 109 Homework v3 1.5 pt Scientific notation, s.f., & Lewis Structure, etc. Fall 12 Due Wednesday, 10/17/12 at 5 PM. 1. (0.5 pt) a) Convert the following into scientific notation, indicate the number of significant figures and the least (farthest to the right) significant place: 7,004 # of s.f. 0.00163 # of s.f. Sig place farthest to right Sig place farthest to right b) Convert the following into standard notation: 8.00 x 103 2.9515 x 10!1 2. (0.2 pt) Indicate the number of p+, e!, and valence e! for the following. Then draw Lewis structures and write “stable” or “not stable” after the structure as would be predicted by the Lewis Octet Rule. # p+ a) P b) Kr2+ # e! # valence e! Lewis Structure Stable??? 3. (0.3 pt) Indicate the total valence e! number and draw Lewis structures for the following molecules or compound ions. For the examples that are larger than diatomic, the central atom in each structure is underlined. a) F2 b) CF2H2 c) NO2! valence e! # Lewis Structure d) (0.1 pt) What is the orbital hybridization of N in NO2! [see 3c), above]. 4. Write an electronic configuration (0.2 pt) for and fill in an energy level diagram of (0.2 pt) a Sr atom. (If you want to save paper, you can write your answer for this on the back of the page you used for Q #1-3.)