Unit 1 Lab - BC Learning Network
advertisement

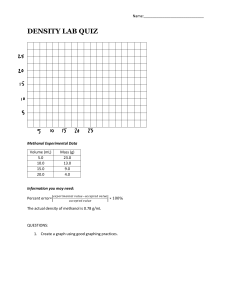
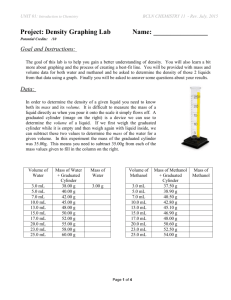
Unit 1 Lab - Density Name:_________________________ Date: _________________________ Purpose: To determine the density of water and methanol using graphing method. Procedure: You will measure the mass of different volumes of a liquid using a 25 mL graduated cylinder, add water and adjust the volume carefully using a medicine dropper. Be sure to read the bottom of the meniscus when determining the volume of the liquid. Uncertainty in the balance is 0.001g and graduated cylinder is 0.5mL. Data and Observations: Mass of 25mL graduated cylinder = 35.00g ± 0.001g Volume of Water (mL) Mass of Water (grams) 3.0 ± 0.5 5.0 7.0 10.0 13.0 15.0 17.0 20.0 23.0 25.0 Mass of water + graduated cylinder 38.00 ± 0.001 40.00 42.00 45.00 48.00 50.00 52.00 55.00 58.00 60.00 Volume of Methanol (mL) 3.0 ± 5.0 7.0 10.0 13.0 15.0 17.0 20.0 23.0 25.0 Mass of methanol + graduated cylinder 37.50 ± 0.001 38.90 40.50 42.80 45.10 46.90 48.00 50.60 52.50 54.00 Mass of methanol(grams) Analysis of results: 3.00 ± 0.002 1.a) Following the rules of good graphing, plot a graph showing mass vs volume for each liquid. You should plot the results for all liquids on the same graph (on entire separate page). Use different colors for each substance. b) Determine the slope of each line rise/run show this on your graph. c) Write the equation of each line in form of Y=mX+b 2. What is the density of each liquid from your graphs? 3. What do you think is the meaning of density and how does this compare with the idea of mass? 4. Use your graph to predict the mass of 6.5mL of methanol? 5. Use your density to calculate the mass of 6.5mL of methanol. Compare your answer to 4. 6. Is every point exactly on the line? Suggest some reason for this. 7. What is the advantage of using the graphing method than just measuring the mass and volume of one sample and calculating results? 8. Indicate the largest source of error and list uncertainty? Conclusion: Summarize your results. 1. List all the values you determined from the lab? 2. Calculate the percentage error for each liquid? Find the correct values for the density of water and methanol by using the internet. Find percentage error using the following formula [Your value- Correct value]/[correct value] x100= percentage error 3. How close are your values to accepted values? Suggest some reason as to why they are not same?