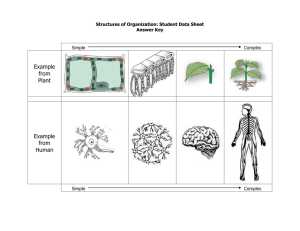
Bohr-Rutherford Diagrams of the First 20 Elements
advertisement

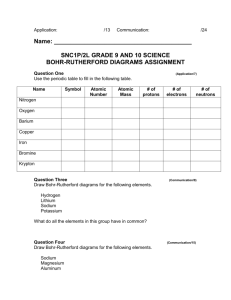
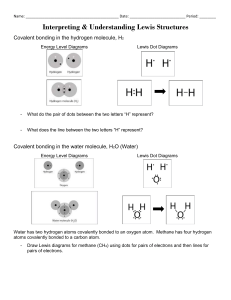
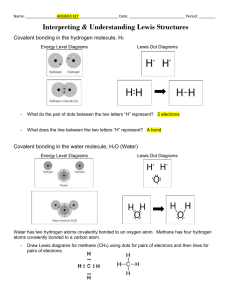
Bohr-Rutherford Diagrams of the First 20 Elements 1 Hydrogen 2 Helium 3 Lithium 4 5 6 7 8 9 10 11 Sodium 12 13 14 15 16 17 18 19 Potassium 20 Instructions: Draw the Bohr-Rutherford diagrams for the atoms of each of the first 20 elements. Show protons (p) and neutrons (n) in the nucleus and use ‘e’ for electrons NOTE: Across a period, with more nuclear charge (+), the diameter shrinks and going down a group, as electron shells are added, the diameter grows. Later we will call this a Trend in Atomic Radius and it will explain why F is more reactive than Cl and why K is more reactive than Li Lewis Dot Diagrams of the First 20 Elements and Common Charges 1 Hydrogen 2 Helium 3 Lithium 4 5 6 7 8 9 10 11 Sodium 12 13 14 15 16 17 18 19 Potassium 20 Instructions: Draw the Lewis dot diagrams for the atoms of each of the first 20 elements, then predict their most common ion (gain or lose electrons to get an octet). eg: Calcium Ca: [Ca2+] NOTE: For a proper Lewis diagram of an ion, we typically show the valence (outer shell) electrons;