Interpreting & Understanding Lewis Structures
advertisement
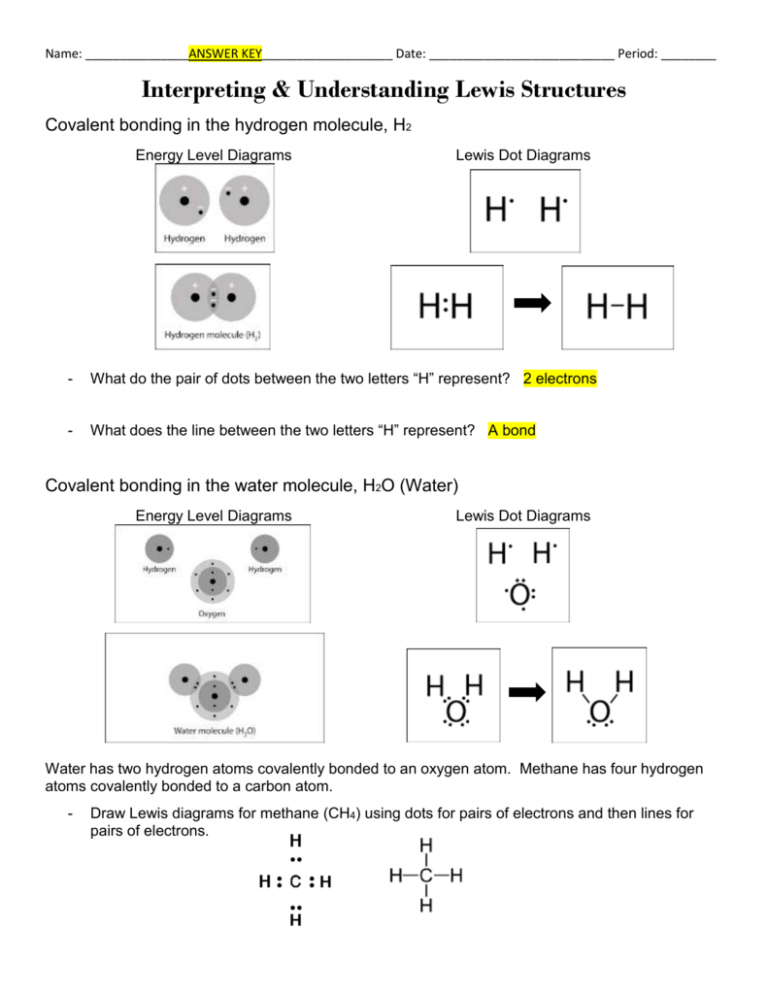
Name: _______________ANSWER KEY___________________ Date: ___________________________ Period: ________ Interpreting & Understanding Lewis Structures Covalent bonding in the hydrogen molecule, H2 Energy Level Diagrams Lewis Dot Diagrams - What do the pair of dots between the two letters “H” represent? 2 electrons - What does the line between the two letters “H” represent? A bond Covalent bonding in the water molecule, H2O (Water) Energy Level Diagrams Lewis Dot Diagrams Water has two hydrogen atoms covalently bonded to an oxygen atom. Methane has four hydrogen atoms covalently bonded to a carbon atom. - Draw Lewis diagrams for methane (CH4) using dots for pairs of electrons and then lines for pairs of electrons. Ionic bonding of sodium chloride, NaCl - In the second dot diagram, why are there no electrons surrounding sodium? Because it gave its electron away - In the final dot diagram of NaCl, the dots between the sodium and chlorine are between the atoms. Are these atoms sharing the electrons? No, chlorine has them all Lewis Structure Practice Guidelines: 1. The octet rule says that atoms “try” to have 8 valence electrons by sharing or transferring electrons to form compounds. *Hydrogen and Helium only have 2 electrons in their “octet” 2. Determine the number of valence electrons 3. Put the atom which can share the most electrons in the middle (think about their individual dot diagrams) 4. Determine and bond the terminal atoms. 5. Remember: Some atoms can share two or three pairs of electrons (usually it is C, N, or O) 6. Hydrogen and fluorine NEVER share two pairs of electrons. HF CH4 PH3 N2 C2Cl6 O2 BF3 NH4+ NO3-