Heating Curve of Water Worksheet: High School Science
advertisement

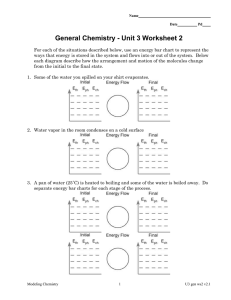
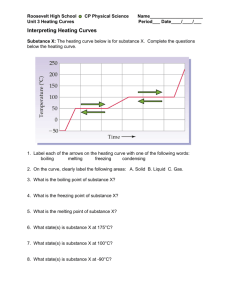
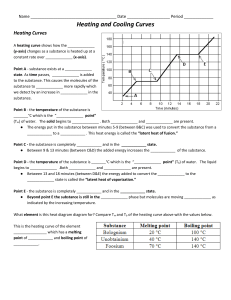
Name: Date: Heating Curve of Water Activity Water molecules have a strong attraction to one another. Because of this property, water has high melting and boiling points and a high specific heat. In this activity, you will plot the heating curve of water and explain what each part of the curve represents at the molecular level. Procedure Use the data in the table to plot a heating curve of temperature versus time for a 180-g sample of water as it is heated at a constant rate from -20°C to 120°C. Draw a best-fit line through the points. Note the time required for water to pass through each segment of the graph. Time and Temperature Data for Water Time Temperature Time Temperature (min) (°C) (min) (°C) 0 1 2 3 4 5 6 7 8 9 10 11 12 -20 0 0 9 26 42 58 71 83 92 98 100 100 13 14 15 16 17 18 19 20 21 22 23 24 25 100 100 100 100 100 100 100 100 100 100 100 100 120 Think Critically 1. What experimental factors would affect the lengths of the flat regions of the graph? 2. What experimental factors would affect the slope of the curve between the flat regions? Name: Date: 3. Analyze each of the five regions of the graph (label them A-E), which are distinguished by an abrupt change in slope. For each segment, indicate what is physically happening to the molecules (for example, boiling), whether it is a change that makes the molecules move faster or farther apart, and if the change in energy is kinetic or potential. A. B. C. D. E. 4. What would the heating curve of ethanol look like? Ethanol melts at -114°C and boils at 78ºC. Sketch ethanol’s curve from -120°C to 90°C.