CHEM 210 Study Guide
advertisement

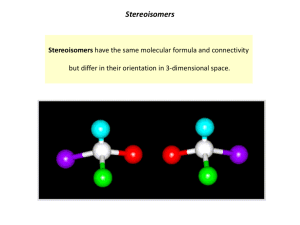
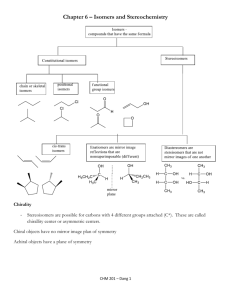
Exam 2 - Study Guide CHEM 210 CHAPTER 4: Alkanes – Nomenclature and Conformations 1. Alkanes a. Know the difference between cyclic and acyclic alkanes and the general formula for each b. Be able to identify carbons as 1o, 2o, 3o or 4o c. Memorize the first 10 n-alkane root names d. Be able to name any alkane or cycloalkane of 10 carbons or less (for its straight chain backbone) e. Be able to draw the structure of any alkane or cycloalkane of 10 carbons or less given the name f. Be able to name and draw structures for other basic functional groups in Chapter 4 Alkenes Alkynes Halides Alcohols Bicyclics 2. Conformational Analysis – Alkanes: a. Know how to draw Newman projections and what they represent b. Be able to draw and identify the various conformations (eclipsed, staggered, gauche, etc.) for the arrangement of groups in a Newman projection c. Given a table of interaction energies, you should be able to generate a potential energy plot vs. dihedral angle for a given rotation through a bond d. Know the definition of torsional strain and steric strain. e. What is a barrier to rotation? Be able to calculate the maximum interaction energy for a set of conformers. 3. Conformational Analysis – Cycloalkanes: a. What is angle strain? What features of cycloalkanes attempt to alleviate this? b. Be able to draw and interconvert the chair and boat forms of cyclohexane c. Be able to identify axial and equatorial positions d. Be able to convert a 2-D Lewis representation to a chair conformer e. Know the definition of steric, torsional and angle strain f. Be able to indicate the axial and equatorial positions of cyclohexane chair conformers g. For mono- or di-substituted ring systems, be able to estimate which isomer or conformer would have the least potential energy (minimize 1,3-diaxial interactions) h. Be able to identify, name and draw cis- and trans-isomers of cycloalkanes CHAPTER 5 – Stereochemistry: 1. Know the classes of isomers and how they differ and are organized a. What is a configuration? What is a stereogenic or chiral center? What are enantiomers? diastereomers? meso compounds? It is safe to say anything in a beige box or in bold would need to be learned! b. Be able to find all of the chiral centers in a molecule c. Be able to draw proper perspective figures for chiral centers (wedge and dash) 2. Know the Cahn-Ingold-Prelog rules for assigning priority about a chiral center a. For a chiral center where a specific configuration is indicated, identify the center as R or S; for an alkane be able to fully name (or draw given the name) a chiral hydrocarbon b. Be able to draw a pair of enantiomers for a chiral center 3. Be able to recognize if a pair of isomers are enantiomers, diastereomers or meso compounds. a. Know the number of stereoisomers possible for a given number of chiral centers; be able to draw a complete set of stereoisomers for a compound with two chiral centers b. Be able to recognize and discuss chirality in cycloalkane systems (we did not cover this in detail in class, as it is an extension of the stereoisomers of acyclic compounds) 4. What is optical activity? How is it calculated? What is specific rotation? How are enantiomers related through optical activity? 5. What is a racemic mixture? 6. How do enantiomers and diastereomers differ in physical properties? Disclaimer – a study guide is simply that – a guide to help you outline and organize your preparation for the exam. There may be material on the exam not explicitly mentioned here that will be used to test your application of knowledge.