Chiral Carbon Atoms
advertisement
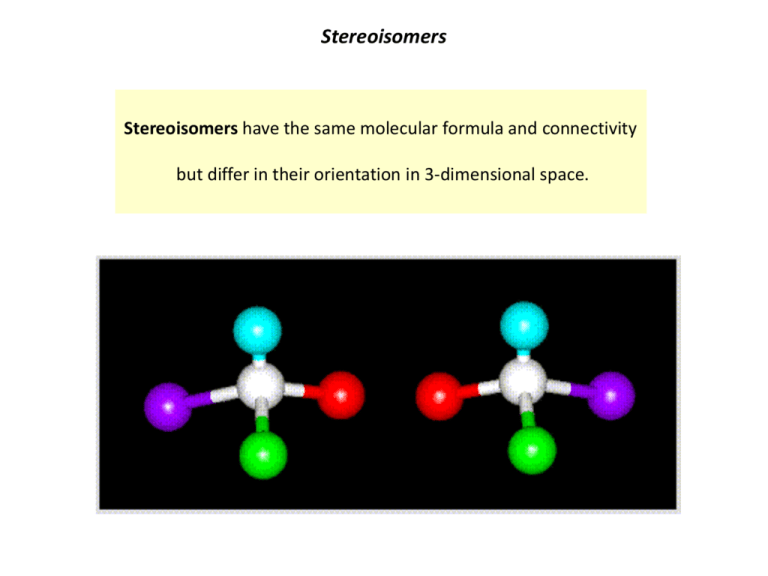
Stereoisomers Stereoisomers have the same molecular formula and connectivity but differ in their orientation in 3-dimensional space. Chiral Carbon Atoms An sp3 hybridized carbon atom with four different substituents is chiral Chiral atoms are also referred to as sterogenic centers Solid wedge – substituent projecting forward Dashed wedge – substituent projecting backward Chiral Molecules If a molecule contains one or more chiral carbon atoms, and it does not have a plane of symmetry, it is a chiral molecule. Achiral molecule Meso compound Chiral molecule Meso compounds contain chiral centers, but have a plane of symmetry so are achiral. Enantiomers Enantiomers are stereoisomers that are non-superimposable mirror images of each other. Molecules with one or more chiral carbon atoms can have enantiomers. (R)-carvone caraway scent (S)-carvone spearmint scent Enantiomers have the same chemical properties (e.g. melting point, boiling point) but can have different biological effects because the body has chiral biomolecules proteins, peptides, carbohydrates, nucleic acids, lipids. Physical Properties of Enantiomers are Identical Except… Identical Enantiomers have identical NMR, IR and UV spectra, densities, melting points and boiling points. Different Enantiomers rotate plane polarized light in opposite directions of each other. (+): Rotation to the right (-): Rotation to the left You can not assign absolute configuration based on optical rotation alone. (R)-enantiomers can be (+) or (-) and (S)-enantiomers can be (+) or (-). Some Drugs Are Marketed as Racemic Mixtures Ibuprofen NSAID Racemic mixture A racemic mixture is a 50:50 mixture of two enantiomers. (S)-Ibuprofen active (R)-Ibuprofen inactive Some Drugs Must be Administered as Single Enantiomers Due to Toxicity of Enantiomer L-Dopa D-Dopa Flip D-Dopa like a pancake, can see it’s the mirror image of L-Dopa L-Dopa Treatment of Parkinson’s disease D-Dopa Toxic Single Enantiomers Are Marketed For Some Drugs That Do Not Have a Toxic Enantiomer Mixture of enantiomers Single enantiomer Celexa® (citalopram oxalate) R- and S- enantiomers antidepressant / antianxiety selective serotonin reuptake inhibitor (SSRI) Lexapro® (escitalopram oxalate) S-enantiomer Considerations of single-enantiomer drugs: 1) Lower dose (only active enantiomer) 2) Potentially fewer side effects 3) More expensive Enantiomeric Compounds Can Have Drastically Different Biological Effects (S)-methamphetamine FDA approved for ADHD, obesity CNS stimulant (euphoria at high doses) (R)-methamphetamine OTC decongestant - Vicks Vapor Inhaler Little or no CNS activity “Levmetamfetamine” listed as active ingredient Enantiomeric Compounds Can Have Drastically Different Biological Effects (R)-thalidomide sedative, antiemetic prescribed for morning sickness 1957-1961 Can not administer thalidomide as a single enantiomer drug because it isomerizes in vivo. (S)-thalidomide potent teratogen Thousands of children born with birth defects As the Number of Chiral Carbons in a Molecule Increases, So Does the Number of Possible Stereoisomers If a molecule has n chiral carbon atoms, there are 2n possible steroisomers * may be fewer due to symmetry 2 chiral carbons 22 = 4 steroisomers * * Diasteromers Diastereomers are stereoisomers that are not enantiomers. Molecules with two or more chiral carbon atoms can have diasteromers. Ephedrine Pseudoephedrine Stimulant, appetite suppressant, decongestant Decongestant, stimulant On a Side Note… Pseudoephedrine and Ephedrine Are No Longer OTC Pseudoephedrine Original formula No longer OTC (S)-methamphetamine FDA approved for ADHD, obesity CNS stimulant (euphoria at high doses) Phenylephrine “New formula” Sudafed Configuration and Conformation Configuration: Changing the configuration of a molecule requires bonds to be broken and reformed. A different configuration is a different molecule. (R)-configuration (S)-configuration Conformation: Changing the conformation of a molecule means rotating about single bonds. Conformations are interconvertible and are the same molecule. (R)-configuration in different conformations