Fluorine & Lithium: An Elementary Love Story Worksheet
advertisement

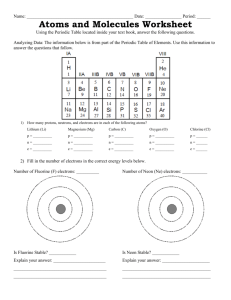
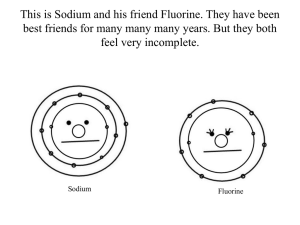
An Elementary Love Story One day a lonely fluorine atom was looking for a “friend”. She decided to join an online dating site, Chemistry.org. On her profile she wrote: Lonely Group 7 Element – wants to meet an interesting metallic element with a view to producing a compound. Questions 1. How many electrons does fluorine have in its outer electron shell? Explain two ways that you can use the Periodic Table to work this out. 2. How many extra electrons does fluorine need to gain to get a full outer electron shell. Explain your answer. Later that day Lithium was browsing the Cemistry.org web site when he happened to see Fluorine’s post. “Wow,” he said, “She sounds like just the element for me.” So he posted a reply. Hi Fluorine – you sound just my type. I have exactly the right number of electrons for you. Why don’t we get together? LOL Li :) P.S Please send me a picture. Questions 3. What group of the Periodic Table is Lithium found in and how many electrons will he have in his outer shell? 4. Draw a picture of Lithium’s electron arrangement that he could send to Fluorine. Fluorine wrote back straight away. Dear Lithium – you have everything I’m looking for in an element. I’m sure that when you give me your outer electron I’ll find you even more attractive. I’ll be so happy to be your bride I’ll change my name from fluorine to fluoride! BFN F Rocketresources Questions 5. Draw a picture of Fluorine’s electron arrangement that she could send back to Lithium . 6. Why will Fluorine find Lithium even more attractive once he gives her his outer electron? 7. What will be the name of the compound they form? 8. Draw a dot cross diagram to show the compound on it’s “wedding day”. 9. What type of chemical bonding does this compound contain? Why is this a good name? 10.Make a list of 4 properties that are typical of this type of compound. Rocketresources