File - StewArt
advertisement

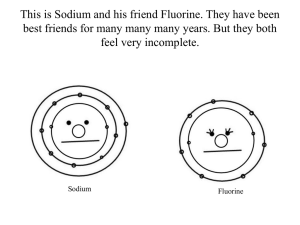
1. Fluorine Atomic Number: 9 Symbol: F Atomic Weight: 18.998403 2. Discovery: Henri Moissan 1886 (France) 3. Word Origin: Latin and French fluere: flow or flux 4. Properties: Fluorine has a melting point of -219.62°C (1 atm), boiling point of -188.14°C 5. Fluorine is a corrosive pale yellow gas. 6. It is highly reactive, participating in reactions with virtually all organic and inorganic substances. 7. Fluorine is the most electronegative element. 8. Metals, glass, ceramics, carbon, and water will burn with a bright flame in fluorine. 9. It is possible that fluorine can substitute for hydrogen in organic reactions. 10. Fluorine has been known to form compounds with rare gases, including xenon, radon, and krypton. 11. Free fluorine has a characteristic pungent odor, detectable. 12. Both elemental fluorine and the fluoride ion are highly toxic. 13. Uses: Fluorine and its compounds are used in producing uranium. 14. Fluorine is used to produce many chemicals, including several high-temperature plastics. 15. The presence of sodium fluoride in drinking water at the level of 2 ppm may cause mottled enamel in teeth, skeletal fluorosis, and may be associated with cancer and other diseases. However, topically applied fluoride(toothpaste, dental rinses) has been shown to help reduce dental caries.