Atoms and Molecules Worksheet: Chemistry Basics
advertisement

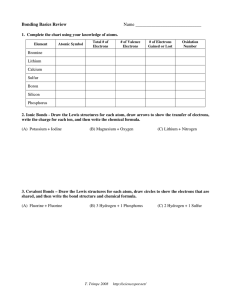
Name: _______________________________________________ Date: ________________ Period: ______ Atoms and Molecules Worksheet Using the Periodic Table located inside your text book, answer the following questions. Analyzing Data: The information below is from part of the Periodic Table of Elements. Use this information to answer the questions that follow. 1) How many protons, neutrons, and electrons are in each of the following atoms? Lithium (Li) Magnesium (Mg) Carbon (C) Oxygen (O) Chlorine (Cl) p = __________ p = __________ p = __________ p = __________ p = ______ n = __________ n = __________ n = __________ n = __________ n = ______ e = __________ e = __________ e = __________ e = __________ e = ______ 2) Fill in the number of electrons in the correct energy levels below. Number of Fluorine (F) electrons: __________ Number of Neon (Ne) electrons: ___________ Is Fluorine Stable? ____________ Is Neon Stable? ____________ Explain your answer: _______________________ Explain your answer: _____________________ _________________________________________ _______________________________________ _________________________________________ _______________________________________ 3) Fill in the chart below. If the atom does not usually ionize, fill in the blank with an X. ELEMENT Sodium ATOMIC MASS NUMBER OF NUMBER OF NUMBER OF NUMBER NUMBER PROTONS NEUTRONS ELECTRONS 11 23 20 20 Calcium Oxygen 8 Chlorine 8 35 Carbon 6 12 Neon 10 20 18 4) Ionic Bond Drawing: Fill in the two tables based on the number of electrons before and after bonding. Drawing Directions a. Add the correct number of electrons to each atom. b. Using an arrow, show the electrons that will be transferred. Before bonding, is lithium and fluorine stable? Explain your answer. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ After bonding, is lithium and fluorine stable? Explain your answer. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________