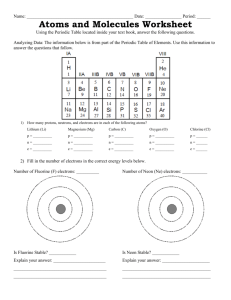
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
advertisement
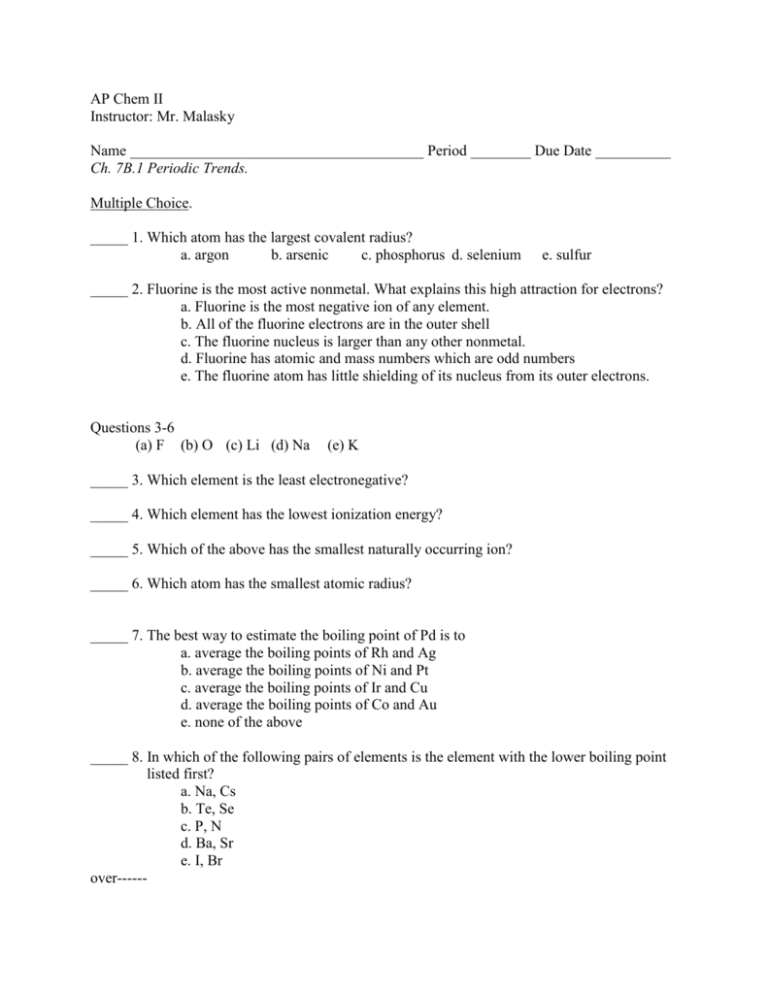
AP Chem II Instructor: Mr. Malasky Name _______________________________________ Period ________ Due Date __________ Ch. 7B.1 Periodic Trends. Multiple Choice. _____ 1. Which atom has the largest covalent radius? a. argon b. arsenic c. phosphorus d. selenium e. sulfur _____ 2. Fluorine is the most active nonmetal. What explains this high attraction for electrons? a. Fluorine is the most negative ion of any element. b. All of the fluorine electrons are in the outer shell c. The fluorine nucleus is larger than any other nonmetal. d. Fluorine has atomic and mass numbers which are odd numbers e. The fluorine atom has little shielding of its nucleus from its outer electrons. Questions 3-6 (a) F (b) O (c) Li (d) Na (e) K _____ 3. Which element is the least electronegative? _____ 4. Which element has the lowest ionization energy? _____ 5. Which of the above has the smallest naturally occurring ion? _____ 6. Which atom has the smallest atomic radius? _____ 7. The best way to estimate the boiling point of Pd is to a. average the boiling points of Rh and Ag b. average the boiling points of Ni and Pt c. average the boiling points of Ir and Cu d. average the boiling points of Co and Au e. none of the above _____ 8. In which of the following pairs of elements is the element with the lower boiling point listed first? a. Na, Cs b. Te, Se c. P, N d. Ba, Sr e. I, Br over------ _____ 9. In which of the following pairs is the first element expected to have a higher electronegativity than the second? a. O, P b. Cs, Rb c. I, Br d. Al, P e. Sb, As _____ 10. A solid element has two valence electrons. That element must be a. a halogen b. a noble gas c. a radioactive element d. an alkali metal e. an alkali earth metal _____ 11. Which of the following is expected to have the largest third ionization energy? a. Be b. B c. C d. N e. Al _____ 12. Which pair of elements is expected to have the most similar properties? a. potassium and lithium b. sulfur and phosphorus c. silicon and carbon d. strontium and barium e. fluorine and iodine