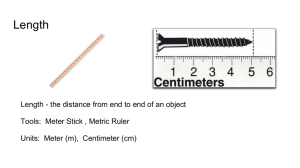
Important points from the density lab…
advertisement
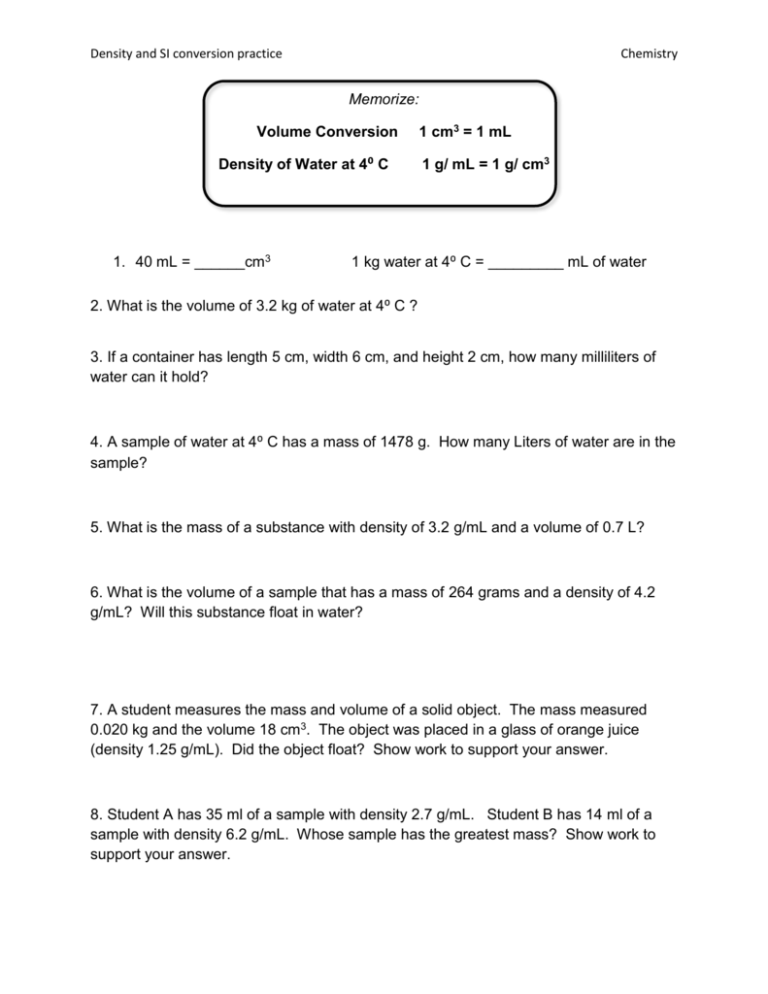
Density and SI conversion practice Chemistry Memorize: Volume Conversion Density of Water at 4⁰ C 1. 40 mL = ______cm3 1 cm3 = 1 mL 1 g/ mL = 1 g/ cm3 1 kg water at 4⁰ C = _________ mL of water 2. What is the volume of 3.2 kg of water at 4⁰ C ? 3. If a container has length 5 cm, width 6 cm, and height 2 cm, how many milliliters of water can it hold? 4. A sample of water at 4⁰ C has a mass of 1478 g. How many Liters of water are in the sample? 5. What is the mass of a substance with density of 3.2 g/mL and a volume of 0.7 L? 6. What is the volume of a sample that has a mass of 264 grams and a density of 4.2 g/mL? Will this substance float in water? 7. A student measures the mass and volume of a solid object. The mass measured 0.020 kg and the volume 18 cm3. The object was placed in a glass of orange juice (density 1.25 g/mL). Did the object float? Show work to support your answer. 8. Student A has 35 ml of a sample with density 2.7 g/mL. Student B has 14 ml of a sample with density 6.2 g/mL. Whose sample has the greatest mass? Show work to support your answer. Density and SI conversion practice Chemistry Important points from the density lab… To find the density of an object, you must measure its _________ and ______________ Part 1: Density of Water We cannot pour water directly onto the triple beam balance to find its mass because it will splash everywhere. Describe how we found the mass of the water. To find the water’s volume, we used the graduated cylinder and then poured the water into a beaker. Why did we do this extra step instead of just measuring the water in the beaker? List at least 3 reasons why the experimental density you found for water did not exactly match the theoretical value of 1 g/ml. Part 2: Density of a Marble Why did we drop the marble into the graduated cylinder when we were trying to find its density? The procedure said to use 50 mL of water in the graduated cylinder. Could we have used 30 mL instead? Explain Since the marble sunk in the graduated cylinder, we know the density of the marble should be greater than _________. Check to make sure your density measurement reflects the fact that the marble sinks. Part 3: Density of the Wood Block Explain how you found the volume of the wood block. Why can’t we drop the block into water and use water displacement to find the volume? Does your density measurement reflect the fact that the wooden block floats?