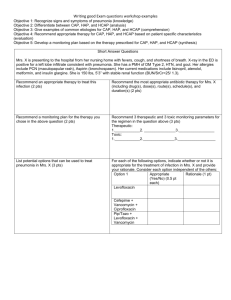
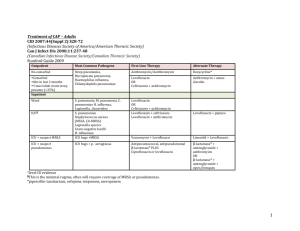
A Comparative Study of Levofloxacin and Ceftriaxone in the
advertisement

A Comparative Study of Levofloxacin and Ceftriaxone in the Treatment of Hospitalized Patients with Pneumonia S. RAGNAR NORRBY1, WOLFGANG PETERMANN2, PAUL ANTHONY WILLCOX3 NORBERT VETTER4 and ERICH SALEWSKI5 From the 1Department of Infectious Disease, University of Lund, Lund, Sweden, 2Innere Abteilung. Brüderkrankenhaus, St. Josef, Paderborn, Germany, the 3Respiratory Clinic, Groote Schuur Hospital, Cape Town, South Africa, the 4Pulmologisches Zentrum der Stadt Wien, Vienna, Austria and the 5Ήoechst Marion Roussel Deutschland, Clinical Development, Frankfurt, Germany A multinational, multicentre, open, randomised study in hospitalised patients with pneumonia compared lerofloxacin 500 mg twice daily with ceftríaxone 4 g i.v. once daily. Levofloxacin patients started on i.v. treatment and switched to oral on d 3-5 of therapy if signs and symptoms had improved. The minimum treatment duration was 5 d, except for treatment failure, and the median 8 d. The primary efficacy analysis was based on the per-protocol assessment of the clinical cure rate determined 2-5 d after the end of treatment in the per-protocol (PP) population (levofloxacin 127, ceftríaxone 139). Of 625 patients enrolled and randomized, 6 received no treatment, giving an intention-to-treat (ITT) population of 619 (levofloxacin 314, ceftríaxone 305). At the clinical endpoint, 2-5 d after the end of treatment, the cure rates for levofloxacin and ceftríaxone were similar in both the ITT (76% and 75%, respectively) and PP (87% and 86%, respectively) populations. Both drugs were well tolerated. Twice-daily levofloxacin 500 mg, either i.v. or as sequential iv/oral therapy, was as effective as i.v. once-daily ceftríaxone 4 g in the treatment of hospitalized patients with pneumonia and offers the advantage of sequential therapy. S. R. Norrby, MD, University of Lund, Department of Infectious Diseases, University Hospital of Lund, S -221 85 Lund, Sweden INTRODUCTION Levofloxacin, a new fluoroquinolone, is the L-isomer of the racemate ofloxacin. The antibacterial action of ofloxacin resides almost entirely in the L-isomer and levofloxacin appears to be twice as active as the racemate ofloxacin in vitro (1). Levofloxacin exerts antibacterial activity via antagonism of the interaction between bacterial DNA gyrase and DNA. Levofloxacin exhibits a broad spectrum of activity, including Gram-positive aerobic organisms such as Streptococcus pneumoniae and Staphylococcus aureus, Gramnegative bacteria such as Escherichia coli, Moraxella catarrhalis, Haemophilus influenzae, Klebsiella pneumoniae and Pseudomonas aeruginosa and intracellular pathogens responsible for atypical pneumonia such as Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae (1-3). Levofloxacin has been shown to be more active against S. pneumoniae in vitro and in vivo in an animal model than either ofloxacin or ciprofloxacin (4, 5). Orally administered levofloxacin is rapidly absorbed, with a bioavailability around 100%. Following oral or intravenous administration it is eliminated relatively slowly, with a terminal half-life of 6-8 h, primarily by renal excretion (6). Levofloxacin is not metabolized to any clinically relevant extent. Most cases of pneumonia are community-acquired (7). Compared with younger patients, community-acquired © 1998 Scandinavian University Press. ISSN 0036-5548 pneumonia (CAP) in the elderly has a greater incidence of co-morbidity, and higher rates of hospitalisation and mortality. Streptococcus pneumoniae, H. influenzae and M. pneumoniae are the most common bacterial pathogens in CAP (8, 9). Staphylococcus aureus and Gram-negative bacteria are recovered from sputum specimens in approximately 310% of cases although their causative role is debatable (7). During the 1980s, M. catarrhalis, Legionella spp. and C. pneumoniae emerged as significant pathogens and a recent study by Márrie et al., in ambulatory patients with CAP, showed that nearly half the cases were due to 'atypical' bacteria (10, 11). Up to 10% of CAP may have a multiple aetiology with S. pneumoniae and H. influenzae being the most common combination. Pneumonia is a serious hospital-acquired infection, particularly amongst critically ill patients. Crude mortality rates range from 50% to 70%, although only one-third of these deaths are directly attributed to the pneumonia (12). Hospital-acquired pneumonia or pneumonia in immunocompromised patients is frequently caused by Gram-negative bacilli such as P. aeruginosa. Anaerobic bacteria are most likely to be found in patients with aspiration pneumonia, lung abscess or empyema. In the hospital environment, the likelihood of colonisation by coliforms may be increased by serious underlying diseases, confinement in an intensive care unit, tracheostomy, use of endotracheal tubes, contaminated respiratory equipment, and antimicrobial treatment which may select resistant organisms. The present study was designed to compare levofloxacin, either i.v. or sequential i.v. to oral, with i.v. ceftriaxone in the treatment of hospitalised patients with pneumonia. Ceftriaxone has been used extensively in patients with pneumonia, either alone or in combination with other antibiotics (7. 13-15). PATIENTS AND METHODS Study design This was a multinational (13 countries), multicentre (64 centres), open, randomised, comparative study in hospitalised patients with pneumonia of levofloxacin (Hoechst AG, Frankfurt. Germany) 500 mg twice daily versus ceftriaxone (F. Hoffmann-La Roche Ltd., Basel, Switzerland) 4 g i.v. once daily. Randomization was centralized using a computerized telephone system (G. H. Besselaar Associates, Belgium) and a 1:1 allocation to levofloxacin or ceftriaxone. Patients in the levofloxacin group were started on i.v. treatment and switched to oral on d 3-5 of therapy, after a minimum of 4 i.v. doses, if clinical signs and symptoms of pneumonia had improved. The total duration of treatment was at the discretion of the investigator although the minimum treatment period was 5 d unless there was a treatment failure. However, it was recommended that treatment be continued for 2 d after fever subsided. The primary efficacy analysis was based on the protocol assessment of the clinical cure rate determined 2-5 d after the end of treatment in the per-protocol population. Three types of assessments were performed according to the study protocol: protocol, conducted by computer (this was the primary assessment); investigator, conducted by the investigator and evaluator-blinded, conducted by an external eval-uator for selected patients using blinded data. The study was conducted in accordance with the Good Clinical Practice guidelines of the European Community and the Declaration of Helsinki. The study protocol was approved by the local ethics committee for each study centre and informed consent was obtained from each patient. Patients Male or female hospitalized patients, aged > 18 y (21 y in parts of Germany), were eligible for inclusion in the study if they had clinical signs and symptoms of bacterial pneumonia, either nosocomial or CAP and chest X-ray findings consistent with the clinical diagnosis of pneumonia. Clinical signs and symptoms included fever, chills, tachypnoea, tachycardia, pleuritic chest pain, cough productive of mucopurulent sputum, leucocytosis, dullness over the involved lobe or segment, diminished breath sounds, bronchial breath sounds, rales and pleural friction rubs. Patients < 65 y of age had to have underlying disease(s) and/or risk factor(s) which increased the risk of acquiring pneumonia or which could complicate the clinical course. Nosocomial pneumonia was defined according to the CDC definition (16). CAP was defined as an infection that occurred outside the hospital environment. Exclusion criteria included: pneumonia strongly suspected to be caused by mycoplasma, chlamydia or legionella; history of epilepsy: pregnancy or nursing mothers: severe hepatic disease; renal impairment (creatinine clearance < 20 ml/min); antibiotic treatment for > 24 h in the 5 d prior to study entry; azithromycin in the 7 d prior to study entry; ofloxacin or ceftriaxone for this infectious episode; any invcstigational drug in the 4 weeks prior to study entry or during the study period; hypersensitivity to fluoroquinolones or ceftriaxone. Efficacy analysis The intention-to-treat (ITT) analysis was performed on the clinical response in all patients who received at least one dose of study drug. The per-protocol analysis, which was the primary analysis, was performed on the clinical and bacteriological response in all patients with clinical signs and symptoms of pneumonia, consistent X-ray findings and bacteriologically proven infection as treated, excluding major protocol violators. The clinical response was assessed at endpoint (2-5 d after the end of therapy), at change from i.v. to oral levofloxacin and at follow-up (14-21 d after the end of therapy). The clinical response was defined as: cure (all infection-related signs and symptoms disappeared or returned to preinfection state and chest X-ray findings at least improved; at least one infectionrelated sign or symptom had improved, follow-up chest X-ray had at least improved or was unchanged and no subsequent antibiotic treatment was started); failure (all infection-related signs and symptoms unchanged or worsened; patient developed new clinical findings consistent with active infection which did not improve or disappear at clinical endpoint; patient died due to the infection; study drug discontinued due to clinical and/or bacteriological treatment failure; one or more antibiotics were added to the study drug due to treatment failure) or indeterminate (circumstances precluded classification as cure or failure e.g. missing follow-up data, premature discontinuation for nonefficacy-related reasons or major protocol violation (incorrect entry diagnosis; insufficient treatment duration; non-compliance; antibiotic pre-treatment; additional antibiotic treatment; clinical post-treatment evaluation missing or out of time window); patient received a subsequent antibiotic for an infection other than the indication). The bacteriological response was evaluated from the clinical endpoint culture (2-5 d after the end of treatment) as: satisfactory (eradication or presumed eradication [negative culture] of pathogen; colonization with a different pathogen without any clinical evidence of infection); unsatisfactory (persistence of baseline pathogen; relapse; superinfection; eradication and reinfection; persistence and reinfection; presumed persistence; resistance) or indeterminate (lack of subsequent culture due to withdrawal, death etc.; treatment with a systemic antibiotic in addition to the study drug). Sputum cultures were set up within 48 h before the start of treatment, on d 2 or 3 of therapy and at each efficacy evaluation for the isolation, identification and susceptibility testing of the responsible pathogen(s). In addition, at least 2 sets of blood cultures (aerobic and anaerobic) were set up prior to the start of treatment. Blood cultures were repeated after 48 h treatment in patients with persistent fever but were optional in patients whose temperature was < 37.8°C. Statistical analysis The primary efficacy evaluation was based on the clinical cure rate in the per-protocol population with bacteriologically proven infection at the clinical endpoint. Assuming a success rate of 80% in both treatment arms and a δ of 15% (maximum difference between treatments to be accepted as equivalent), 125 evaluable patients per group were needed to provide a 84.6% chance of showing equivalence (power = 84.6%) by calculating a 2-sided 95% confidence interval (CI) for the difference in cure rates (17. 18). If the upper bound was greater than 0 and the lower bound greater than — 0.15, levofloxacin was considered to be as effective as ceftriaxone. The Wilcoxon rank test was used to evaluate the time between start of treatment and discharge from hospital. Patients who died in hospital were assigned the longest duration of time observed for any patient. It was assumed that 33% of the patients treated would be evaluable for efficacy and, therefore, a total of 750 patients would be required. However, the study was closed with < 750 enrolled patients because the evaluability during the trial was 46%. RESULTS Fig. 1 shows the distribution of patients over the different populations for analysis. 625 patients were enrolled and randomized (619 treated) at 64 study centres in 13 countries. No treatment was given in 6 cases, giving an ITT population of 619 patients, 314 assigned to levofloxacin and 305 to ceftriaxone. Patient demographics were similar in both treatment groups, with 65% of patients being male. The median age was 65 y (range 18-94 y), 51% (315/619) were elderly (65 and 80% (495/619) were Caucasian. The median weight was 66 kg (range 36-129 kg) and the median body mass index was 23.5 kg/m 2 (range 13-51 kg/m). Forty per cent (247/619) were ex-smokers and 34% (209/619) were non-smokers. For 94% (582/619) of patients, the diagnosis was CAP and nosocomial pneumonia was diagnosed in the remaining 6% (37/619). A history of disease and concomitant illnesses was reported in 93% of patients (575/619), with respiratory (330 patients, 53%) and cardiovascular (276 patients, 45%) diseases the most common. Chronic obstructive airways disease (198 patients, 32%) and emphysema (87 patients, 14%) were the most common respiratory diseases. Drug/alcohol abuse was reported in 14% of patients. One hundred and thirty-three patients (21%) had an APACHE II score > 15. Bronchopneumonia was diagnosed in 40% (249/619), lobar pneumonia in 54% (336/619) and in 6% of patients (35) the type of pneumonia was not specified. At admission, disease severity was assessed as moderately severe in 70% (436/619) and severe in 20% (125/619) of patients. Pretreatment with systemic antibiotics during the 5 d before study onset was reported for 25% of patients (154/619) and 96% of patients received at least one concomitant medication during the study. The median duration of treatment was 9 d for levofloxacin and 8 d for ceftriaxone patients in the ITT population (NS). A total of 89 patients [45/319 (14%) levofloxacin, 44/306 (14%) ceftriaxone] did not complete the study and were withdrawn for various reasons (Table I). More patients in the ceftriaxone group (χ 2 = 3.9, p = 0.05) (22) withdrew due to clinical failure than in the levofloxacin group (14). Sequential treatment, i.e. change from i.v. to oral, was administered in 274/314 (87%) levofloxacin patients with a median duration of 4 d on i.v. and 5 d on oral treatment. The cure rate for levofloxacin (76%) was similar to that for ceftriaxone (75%) (Table II). The cure rates in both treatment groups were lower than in the per-protocol population due to the higher number of indeterminate cases (mainly due to protocol violations). Levofloxacin was as effective as ceftriaxone (95% Cl 5.7%; + 7.8%). The per-protocol population, patients with a proven bacteriological infection at baseline (isolation of a responsible pathogen from a baseline culture) excluding major protocol violators (40 levofloxacin, 26 ceftriaxone), consisted of 272 patients (130 levofloxacin, 142 ceftriaxone), 44% of the ITT population. The clinical response was indeterminate in 6 patients and, therefore, the total analysed was 266 (127 levofloxacin, 139 ceftriaxone). At the clinical endpoint, the cure rate for levofloxacin (87%) was similar to that for ceftriaxone (86%) and the 95% Cl indicates that levofloxacin was as effective as ceftriaxone (Table II). Clinical response at endpoint was also assessed by the investigator. Cure rates for the 2 drugs were similar for the ITT population (levofloxacin, 87%; ceftriaxone, 85%) and the per-protocol population (levofloxacin, 87%; ceftriaxone, 89%). The protocol and investigator assessments of clinical response were in agreement for 456 of 502 patients (91%) in the ITT population and for 225 of 240 patients (94%) in the per-protocol population who had both assessments. 236 patients were selected for assessment by an external, blinded evaluator, of which two-thirds were all the patients who had differing outcomes in the protocol (i.e. by computer) and investigator assessments. The remaining third were patients randomly selected from those classified as cured in both of these assessments. The protocol and evaluator-blinded assessments of clinical response were in agreement in 75% (177/236) of ITT patients and 85% (75/88) of per-protocol patients. A total of 351 bacterial isolates, considered responsible for infection by the investigator, were isolated at baseline. The most common pathogens (> 5 isolates) are shown in Table III. The majority of the pathogens isolated were susceptible to both study drugs but 3/332 (1%) tested isolates were resistant to levofloxacin (I S. pneumoniae and 2 P. aeruginosa) and 17/330 (5%) were resistant to ceftriaxone (10 P. aeruginosa, 1 P. fluorescens, 1 Pseudomonas spp., 1 H. influenzae, I K. oxytoca, 1 Enterococcus spp., 1 E. faecalis and 1 Enterobacter cloacae). Similar results were found for isolates of patients assigned to each treatment group (levofloxacin, 1/157, l%; ceftriaxone, 8/174, 5%). In the per-protocol population, 26 (20%) levofloxacin patients and 30 (21%) ceftriaxone patients had bacteraemia. In the per-protocol bacteriological efficacy analysis at clinical endpoint, 61/130 levofloxacin patients and 57/142 ceftriaxone patients had an indeterminate bacteriological response and, therefore, the total number of patients analysed for bacteriological response was 69 and 85, respectively. Of the patients analysed, 57/69 levofloxacin (83%) and 71/85 ceftriaxone (83%) patients had a satisfactory bacteriological response. Persistent or presumed persistent pathogens were the main reason for an unsatisfactory response (levofloxacin 10, ceftriaxone 11). The percentage difference in the comparison of the two treatments was 0.9% and the 95% Cl (- 12.8%; + 11.0%) indicates that levofloxacin was as effective as ceftriaxone. The bacteriological and clinical responses were in agreement for 147/153 patients (96%) who had evaluable data for both assessments. Four patients had a satisfactory bacteriological response but a failed clinical response (levofloxacin 2, ceftriaxone 2) and 2 patients, both ceftriaxone, had an unsatisfactory bacteriological response but a satisfactory clinical response (cure). The remaining patients had an indeterminate bacteriological or clinical response, including 105 patients with a clinical response of cure but an indeterminate bacteriological response (levofloxacin 56, ceftriaxone 49). Eradication rates for the most common pathogens are shown in Table IV. The overall eradication rates were equal for both treatments (87%). The eradication rate for levofloxacin was greater than for ceftriaxone for Gram-negative pathogens (96% versus 88%) but lower for Gram-positive pathogens (76% versus 85%). However, the number of pathogens per treatment group was low relative to the number of patients treated and this must be taken into account when comparing these rates. There were 5 pathogens which persisted at clinical endpoint in 4 patients [levofloxacin: 3 (S. pneumoniae, Group A beta-haemolytic streptococcus and S. aureus) in 2 patients; ceftriaxone: 2 (P. aeruginosa and Acinetobacter baumannii) in 2 patients]. Only the P. aeruginosa isolate in the ceftriaxone group was resistant to the study drug in the post-treatment culture. 95 new pathogens (levofloxacin 35, ceftriaxone 60) were isolated in the ITT population during the study. Gram-negative isolates were more prevalent in the ceftriaxone group (30 vs 9). 39 of the 57 pathogens tested (68%) were susceptible to the assigned study drug. The clinical response of patients designated failure or indeterminate at clinical endpoint was carried over to the follow-up assessment. Patients who received subsequent antibiotics for clinical or bacteriological failure from d 6 post-treatment onwards were classed as failures. For both treatment groups and both populations considered, cure rates at follow-up were approximately 3-5% lower than at clinical endpoint (Table II). In the ITT population, 274/314 patients (87%) switched from i.v. to oral levofloxacin. Of these, 217 patients (79%) changed on d 2-5 of i.v. treatment and the median time to change was d 4 of therapy. The clinical response at time of change was determined as cure in 66% of patients (164 of 250 analysed) and improved in 32% (80). Similar results, 64% and 32%, respectively, were obtained in the per-protocol population analysis. No significant difference in length of hospital stay was detected between treatment groups, with a median duration of 12 d for both groups in the ITT population and 11 d for the levofloxacin group and 13 d for the ceftriaxon group in the per-protocol population. The safety profile of levofloxacin was similar to that of ceftríaxone and both drugs were well tolerated. Adverse events were reported in 334/619 patients (169/314 [54%] levofloxacin, 165/305 [54%] ceftriaxone). The most frequently affected body systems were: body as a whole, cardiovascular system, digestive system, metabolic and nutritional system, nervous system and respiratory system (see Table V). The incidence of adverse events possibly associated with study medication was 68/314 (22%) for levofloxacin and 79/305 (26%) for ceftriaxone (see Table V). There were no significant differences between the treatment groups. Serious adverse events (see Table VI), irrespective of relationship to study drug, were reported in 55/314 levofloxacin patients (18%) and 52/305 ceftriaxone patients (17%). Frequencies were generally similar apart from cardiovascular events, which occurred more frequently in the levofloxacin group (28/314, 9% vs 14/305, 5%). Events which contributed to this difference were heart failure (7 levofloxacin, 4 ceftriaxone); congestive heart failure (3, 0); arrhythmia (2, 0) and tachycardia (2, 0). None of these events were considered to be drug related and all were thought to be due to pre-existing disease. Study treatment was permanently discontinued due to adverse events in 15/314 levofloxacin patients (5%) and 20/305 ceftriaxone patients (7%). There were 43 deaths among the patients who were randomised and treated: 21/314 (7%) levofloxacin and 22/ 305 (7%) ceftriaxone patients. Fifteen deaths occurred during treatment (7 levofloxacin, 8 ceftriaxone), 14 posttreatment to 14-d follow-up (10 levofloxacin, 4 ceftriaxone) and 14 after the 14-d follow-up (4 levofloxacin, 10 ceftriaxone). Cause of death was related to the patients' severe pre-existing conditions and none were considered related to the study drug. There was no significant difference between the death rates in both treatment arms. DISCUSSION The results indicate that levofloxacin, either i.v. or as sequential iv/oral treatment, was as clinically effective as i.v. ceftriaxone in the treatment of hospitalised patients with pneumonia (cure rates of 87% and 86%, respectively, in the per-protocol analysis at clinical endpoint). The study population, pneumonia assessed as moderately severe in 70% and severe in 20% of patients at admission, and the most common baseline pathogens isolated, S. pneumoniae (36%), H. influenzae (21%) and M. catarrhalis (8%), was representative of a hospital population with pneumonia. Notably, 7% of the patients died due to their infections and/or underlying conditions. As expected, cure rates in the ITT population were lower but equally so for both treatment groups so the conclusion for the ITT and per-protocol populations was the same. Equivalence of treatment was further confirmed by comparison of the investigator and protocol assessments and by an evaluator-blinded assessment. Clinical cure rates at follow-up were also similar for both treatment groups in the per-protocol population (83% for levofloxacin and 81% for ceftriaxone). There have been relatively few comparative studies on the efficacy of fluoroquinolone monotherapy in pneumonia, particularly in patients with moderately severe or severe pneumonia. This is probably due to the poor activity of older fluoroquinolones, such as ofloxacin and ciprofloxacin, against S. pneumoniae, the most common pathogen. Plouffe et al. showed that ofloxacin was as effective as standard therapy (ß-lactam alone or plus a macrolide) in patients hospitalized for CAP (clinical success rate 92% and 87%, respectively) (19). A comparison of ciprofloxacin and imipenem-cilastin, in 405 patients with severe pneumonia, mainly of nosocomial origin, obtained a good clinical and bacteriological response in approximately 70% of patients (20). In a comparative study of oral sparfloxacin as monotherapy versus oral amoxycillin plus ofloxacin as combination therapy in 211 hospitalized patients with CAP. the overall efficacy rates were 92% and 82%, respectively (21). Patients in this study were either elderly or had failed a previous course of antibacterial therapy and the main pathogens isolated were S. pneumoniae, H. influenzae, M. pneumoniae, S. aureus and Enterobacteriaceae. In an open, non-comparative study, i.v. or oral levofloxacin once daily was highly effective in mild/moderate and severe CAP and all the pathogens isolated (S. pneumoniae, H. influenzae, S. aureus and C. pneumoniae) were eradicated (22). The results of our study are simitar to those of File et al. who compared i.v. and/or oral levofloxacin once daily with ceftriaxone and/or cefuroxime axetil in an open study in 590 patients, treated in hospital or as outpatients, with mild/moderate or severe CAP (23). However, they showed that levofloxacin was superior to the comparator(s) in terms of both clinical success rates (96% vs 90%) and bacterial eradication rates (98% vs 85%). In our study, the baseline pathogens were generally more often susceptible to levofloxacin than to ceftriaxone. Only 0.9% (3/332) were resistant to levofloxacin compared to 5.2% (17/330) resistant to ceftriaxone, this difference being mainly due to ceftriaxone-resistant Gram-negative pathogens such as P. aeruginosa. However, this difference did not affect the overall bacteriological success rates which were similar for both treatments (levofloxacin 84%, ceftriaxone 83%). Superinfections were rare, 8 in 7 patients (levofloxacin 3, ceftriaxone 4), and there were no reinfections. Both drugs were well tolerated and the majority of adverse events were associated with the severe course of the infectious disease itself. The difference in the incidence of cardiovascular serious adverse events between the levofloxacin and ceftriaxone groups was attributable to the patients' underlying disease and was not related to the study drug. The mortality rate was similar in both treatment groups. The incidence of possible drug-related events, which is higher than in some studies, may in part be due to the study protocol requiring a possible rather than a probable causal relationship to the study drug and/or the severity of the patients' underlying disease (19, 23). Levofloxacìn offers the advantage over ceftriaxone of sequential or switch therapy from i.v. to oral without the need to change the treatment drug. The results of this study indicate that sequential i.v. to oral levofloxacin was as effective as i.v. ceftriaxone. This provides an important benefit in terms of treatment costs. There is a growing pressure in most countries to limit the rise in healthcare costs which, in the hospital environment, has resulted in a trend towards reducing the length of time a patient spends in hospital and the use of more cost-effective treatments. As part of these changes, there is increasing use of switch, therapy due to the advantages which oral administration offers over i.v. therapy. These include reduced complications from i.v. devices, reduced hospital stay and reduced treatment costs (24-26).Several studies have reported the economic benefits of switching from i.v. to oral treatment. Salewski et al. showed a reduction in costs of approximately 50% when switching from i.v. to oral treatment with ofloxacin or ciprofloxacin in a multi-centre hospital study in patients with a variety of infections (27). Garber et al. reported an annual saving of nearly SC60000 in 1 hospital due to switch therapy (28). In a review of the cost-effectiveness of switch therapy, Jewesson reported savings of approximately SC85000 over a 4-y period at the Vancouver General Hospital (29). In conclusion, the results of this study show that, in hospitalized patients with pneumonia, twice-daily levofloxacin 500 mg, either i.v. or as sequential iv/oral therapy, was as effective as i.v. once-daily ceftriaxone 4 g. ACKNOWLEDGEMENTS This study was supported by a grant from Hoechst Marion Roussel. The authors would like to acknowledge the contribution of the following members of the International Study Group—Argentina: Ambasch G, Ariza HA, Jasovich A. Mingrone HO, Montero JM, Piovano CF, Salvarezza CR; Australia: Looke D; Belgium: Claessens J-J, Gepts L, Jordens P; France: Bernard JP, Boutin C. Brouqui PL, Laaban J-P, Muir JF, Weitzenblum E; Germany: Altrogge G, Berger K, Beyer JH, Bodmann K, Ferünz R, Freytag F, Geier FP. Greiner A, Henrich K, Herms W. Janzik U. Kirsch WD, Matthys H, Mõller A, Sell G. Simon B, Thai W, Vallee D, Weiss R, Wiesner B; Ireland: Clancy LJ. O'Neill S; Italy: Clini V. Vagliasindi M, Velluti G; The Netherlands: Maesen F; South Africa: Heynecke ML, Irusen EM, Mpe MJ, Naude GE, Theron W, van Aswegen GL, van Zyl L, Wood R; Spain: Garcia Bragado F, Pigrau Serrallach C, Segura F; Switzerland: Bernasconi E, Braendli O, Frei A. Vìlliger B; UK: Britton MG, Murphy PG. OΉickey SP. REFERENCES 1. Davis R, Bryson HM. Levofloxacin. A review of its antibacte rial activity, pharmacokinetics and therapeutic efficacy. Drugs 1994; 47: 677-700. 2. Barry AL, Fuchs PC, Allen SD. Brown SD, Jorgensen JH, Tenover FC. In-vitro susceptibility of Streptococcus pneumo niae to the D- and L-isomers of ofloxacin: interpretive criteria and quality control limits. J Antimicrob Chemother 1996; 37: 365-9. 3. Fu KP, Lafredo SC, Foleno B, Isaacson DM, Barrett JF, Tobia AJ, Rosenthale ME. In vitro and in vivo antibacterial activities of levofloxacin (L-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother 1992; 36: 860-6. 4. Klugman KP, Capper T. Levofloxacin in-vitro activity and synergistic activity in combination with other antibacterials against antibiotic-resistant S. pneumoniae and selection of resistant mutants. Proceedings of 35th ICAAC, San Francisco, USA 1995; Abstract E9. 5. Markus A, Isert D, Klesel N, Seibert G. Killing activity of levofloxacin and ciprofloxacin against Streptococcus pneumo niae in vitro and in vivo. Proceedings of 35th ICAAC, San Francisco. USA 1995; Abstract E30. 6. Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet 1997; 32: 101-19. 7. Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med 1995; 333: 1618-24. 8. Pass RJ. Aetiology and treatment of community-acquired pneumonia in adults: an historical perspective. J Antimicrob Chemother 1993; 32 Suppl A: 17-27. 9. Finch RG. The role of new quinolones in the treatment of respiratory tract infections. Drugs 1995; 49, Suppl 2: 144-51. 10. Fang G-D, Fine M, Orloff J, Arisumi D, Yu VL, Kapoor W. et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy: a prospective multicenter study of 359 cases. Medicine 1990; 69: 307-16. 11. Marrie TJ, Peeling RW, Fine MJ. Ambulatory patients with community-acquired pneumonia: the frequency of atypical agents and clinical course. Am J Med 1996: 101: 508-15. 12. Craven DE. Prevention of hospital-acquired pneumonia: mea suring effect in ounces, pounds, and tons. Ann Intern Med 1995; 122: 229-31. 13. Mangi RJ. Peccerillo K.M, Ryan J, Berenson C, Greco T, Thornton G. Andriole VT. Cefoperazone versus ceftriaxone monotherapy of nosocomial pneumonia. Diagn Microbiol In fect Dis 1992; 15: 441-7. 14. Hirata-Dulas CA. Stein DJ, Guay DR. Gruninger RP, Peter son PK. A randomized study of ciprofloxacin versus ceftriax one in the treatment of nursing home-acquired lower respiratory tract infections. J Am Geriatr Soc 1991; 39: 97985. 15. The British Thoracic Society, London. Guidelines for the management of community-acquired pneumonia in adults ad mitted to hospital. Br J Hosp Med 1993; 49: 346-50. 16. Garner JS, Jarvis WR, Emori TG, Horan TC. Hughes JM. CDC definitions for nosocomial infections 1988. Am J Infect Control 1988; 16: 128-40. 17. Harkins RD. Albrecht R. Design and analysis of clinical trials for anti-infective drug products. Drug Infect J 1990; 24: 21324. 18. Makuch R, Simon R. Sample size requirements for evaluating a conservative therapy. Cancer Treat Rep 1978; 62: 1037-40. 19. Plouffe JF, Herbert MT, File TM Jr, Baird I. Parsons JN, Kahn JB, Rielly-Gauvin KT, and The Pneumonia Study Group. Ofloxacin versus standard therapy in treatment of community-acquired pneumonia requiring hospitalization. Antimicrob Agents Chemother 1996; 40: 1175-9. 20. Fink MP, Snydman DR, Niederman MS, Leeper KV, Johnson RJ, Heard SO, Wunderink RG, Caldwell JW, Schentag JJ, Siami GA, Zameck RL, Haverstock DC, Reinhart HH, Echols RM, and The Severe Pneumonia Study Group. Treatment of severe pneumonia in hospitalized patients: Results of a multicenter, randomized double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastin. Antimicrob Agents Chemother 1994; 38: 547-57. 21. Portier H. May TH, Proust A. and The French Study Group. Comparative efficacy of sparfloxacin in comparison with amoxycillin plus ofloxacin in the treatment of community-ac quired pneumonia. J Antimicrob Chemother 1996; 37, Suppl A: 83-91. 22. Fogarty CM. A noncomparative study to evaluate the safety and efficacy of levoñoxacin in the treatment of community-ac quired pneumonia in adults. Proceedings of 19th ICC, Mon treal, Canada 1995. 23. File TM. Segreti J, Dunbar L, Player R, Kohler R, Williams RR, et al. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community-acquired- pneumonia. Antimicrob Agents Chemother 1997; 41: 1965-72. 24. Conly JM, Low DE. Intravenous to oral antimicrobial stepdown therapy: improving the quality of care and reducing costs. Can J Infect Dis 1995; 6 Suppl A: A3-5. 25. Louie TJ. Intravenous to oral stepdown antibiotic therapy: another cost-effective strategy in an era of shrinking health care dollars. Can J Infect Dis 1994; 5 Suppl C: C45-50. 26. Quintiliani R, Crowe HM, Nightingale C. Transitional antibi otic therapy. Can J Infect Dis 1995; 6 Suppl A: A6-10. 27. Salewski E, Bassaris HP, Calangu S. Kitzes R;Kosmidis J, Raz R, Kompan L. Cost-minimisation analysis of sequential treatment with ofioxacin or ciprofloxacin in hospitalised pa tients. Pharmacoecon 1997; 11: 359-66. 28. Garber GE, McLean W, Ticrney M, Page D. Reducing antibi otic costs; the Ottawa General Hospital experience of cost cutting, including intravenous to oral stepdown. Can J Infect Dis 1995; 6, Suppl A: 17A. 29. Jewesson P. Cost-effectiveness and value of an i.v. switch. Pharmacoecon 1994; 5 Suppl 2: 20-6.