Aspirin Post-Lab Questions
advertisement
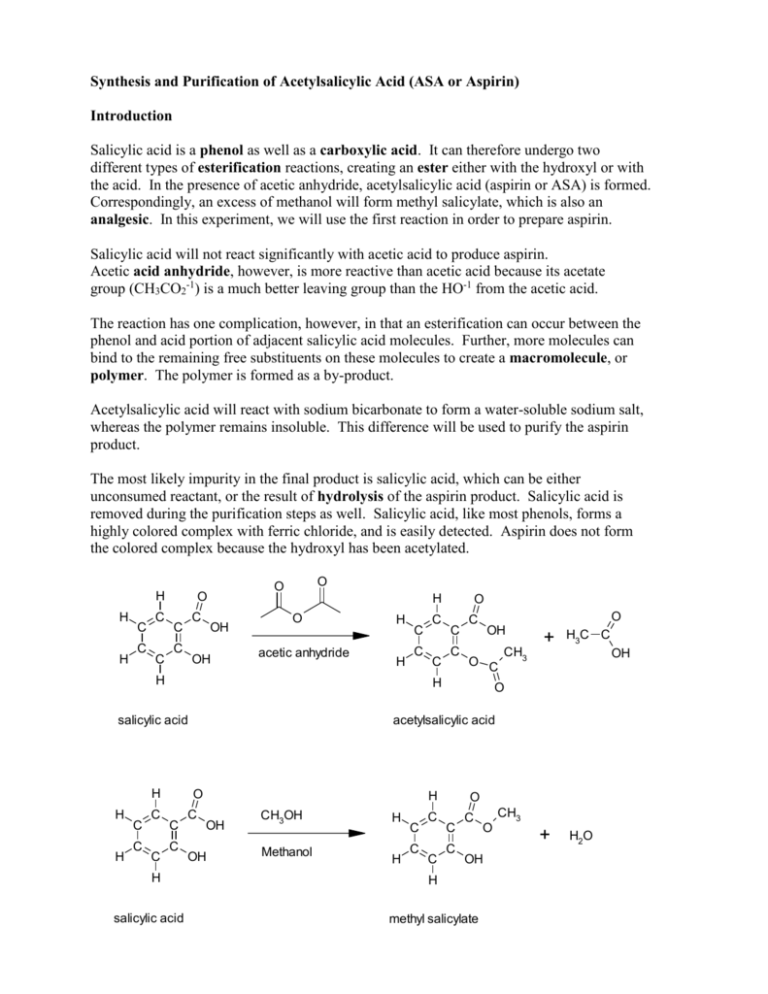
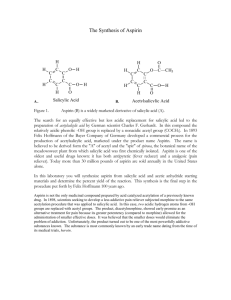
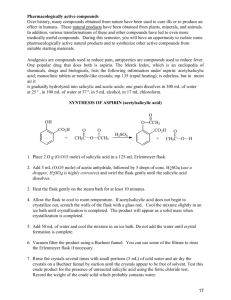
Synthesis and Purification of Acetylsalicylic Acid (ASA or Aspirin) Introduction Salicylic acid is a phenol as well as a carboxylic acid. It can therefore undergo two different types of esterification reactions, creating an ester either with the hydroxyl or with the acid. In the presence of acetic anhydride, acetylsalicylic acid (aspirin or ASA) is formed. Correspondingly, an excess of methanol will form methyl salicylate, which is also an analgesic. In this experiment, we will use the first reaction in order to prepare aspirin. Salicylic acid will not react significantly with acetic acid to produce aspirin. Acetic acid anhydride, however, is more reactive than acetic acid because its acetate group (CH3CO2-1) is a much better leaving group than the HO-1 from the acetic acid. The reaction has one complication, however, in that an esterification can occur between the phenol and acid portion of adjacent salicylic acid molecules. Further, more molecules can bind to the remaining free substituents on these molecules to create a macromolecule, or polymer. The polymer is formed as a by-product. Acetylsalicylic acid will react with sodium bicarbonate to form a water-soluble sodium salt, whereas the polymer remains insoluble. This difference will be used to purify the aspirin product. The most likely impurity in the final product is salicylic acid, which can be either unconsumed reactant, or the result of hydrolysis of the aspirin product. Salicylic acid is removed during the purification steps as well. Salicylic acid, like most phenols, forms a highly colored complex with ferric chloride, and is easily detected. Aspirin does not form the colored complex because the hydroxyl has been acetylated. H H H C C C C C C C O O O H OH OH O acetic anhydride H H C C H H H C C C C C C C O C OH O C H salicylic acid H C O C H salicylic acid + H3C C OH O acetylsalicylic acid O C CH3 C H OH OH CH3OH Methanol H H C C C C O C C C O OH H methyl salicylate CH3 + H2O Background Reading McMurry, J., Organic Chemistry, 8th Ed., pp 830 and 835 (7th Ed, pp 802 & 806-7). J. Beran, Lab Manual for Principles of General Chemistry, 9th and 8th Ed., Experiment 19, pg 231. Key Words phenol, carboxylic acid, ester, acid anhydride, macromolecule Compound, Reaction, and Yield Data Provide systematic names for the reactant and product in the substance section. Provide tabulated and experimental melting ranges for product. Report mass and moles for the reactant and product, and calculate yield % on a molar basis. Mechanism The mechanism is called nucleophilic acyl substitution. It is similar, but not identical, to the hydrolysis on pg 830 in McMurry 8e. The electrophile is an acid anhydride, not an acid chloride. The entering nucleophile is salicylic acid (its phenol O), not water. On the resulting tetrahedral intermediate, the H from salicylic acid moves to the middle O on the anhydride. Finally, the leaving group is acetic acid, rather than chloride. No base is involved. Provide structures of all intermediates in your lab report. Substances 2.0 g salicylic acid 5.0 mL acetic anhydride 5 drops concentrated H2SO4 25 ml saturated NaHCO3(aq) 3.5 mL concentrated HCl Apparatus one 125-mL Erlenmeyer flask 70-mm filter paper and Buchner funnel 250-mL or 500-ml vacuum flask Procedure Part A: Synthesis 1. Weigh 2.0 g of salicylic acid crystals. Place in a 125-ml Erlenmeyer flask. 2. In a hood, slowly add 5.0 ml of acetic anhydride and 5 drops of concentrated sulfuric acid to the flask Caution – Concentrated H2SO4 solutions are corrosive and cause acid burns. Carboxylic acid anhydrides are corrosive and extremely hygroscopic. They will cause burns, and they have a strong vinegar-like odor. Use gloves and avoid all contact with skin, eyes, and nose. Perform entire step in fume hood. 3. Swirl the flask gently until the salicylic acid has completely dissolved. If mixture solidifies completely, proceed to step 4. 4. Heat the flask in a boiling water bath (100 oC) for a minimum of 10 minutes. Clamp the flask to a stand so that it does not fall over into the water bath. 5. Allow the flask to cool slightly, and then place in an ice bath to crystallize the acetylsalicylic acid. If necessary, gently scratch the bottom of the flask with a glass rod (to initiate crystal formation on microscopic glass particles). 6. After crystals have formed, add 50 ml of DI water, and cool the mixture in an ice bath. Do not add water until crystallization is complete. Also, place a beaker of DI water in the ice bath and cool to < 5 oC for use in later steps. 7. Collect the product by vacuum filtration using a 70-mm filter paper and Buchner funnel. The filtrate can be used to rinse the Erlenmeyer flask repeatedly until all of the crystals have been collected. 8. Rinse the crystals collected in the funnel with 5 – 10 ml of 5 oC DI water. Then, apply vacuum to the crystals for 30 seconds to remove all of the liquid. Dispose of filtrate in the appropriately labeled waste jar. Part B: Purification 1. Transfer the crude solid to a 150-ml beaker. Add 25 ml of saturated NaHCO3(aq) solution. Stir until all signs of reaction have ceased. (Listen to fizzing.) 2. Vacuum filter the solution to remove all solid polymeric by-product. 3. Retain the filtrate! It contains the product in solution. Dispose of solid in the appropriately labeled waste jar, and discard the filter paper. 4. Place 10 ml of DI water in a 150-ml beaker, and place the beaker in an ice bath. Slowly add 3.5 ml of concentrated hydrochloric acid. Caution – Concentrated HCl solutions and vapors are corrosive and cause acid burns. Use gloves and avoid all contact with skin, eyes, and nose. 5. Carefully, and slowly, pour the filtrate into the acid mixture while stirring. The aspirin should precipitate out of this solution. If not, ensure that the solution is acidic with blue litmus or pH paper. Cool the mixture in an ice bath to complete the crystallization. 6. Vacuum filter to collect the crystals using a weighed 70-mm filter paper in a Buchner funnel. Rinse the beaker and the Buchner funnel with 5 – 10 ml of 5 oC DI water to maximize product yield. Dispose of filtrate in the appropriately labeled waste jar. 7. Dry the product in the oven (60 oC for 15 minutes), then weigh the dried product. Obtain the melting range as well as the decomposition range, which is only slightly higher than the melting range. Dispose of product in the appropriately labeled waste jar, and discard the filter paper. Post-Lab Questions 1. Salicylic acid can be used to create two different types of esters. Using the balanced reactions in the background section, and Figure 21.4 in McMurry (pg 825 in 8e), explain what happens at each of salicylic acid’s two functional groups. 2. Two salicylic acid molecules can bond together when the phenolic OH of one molecule reacts with the carboxylic acid COOH of the other. Repeated reactions can cause a polymer or large macromolecule to be created. Provide balanced reactions that show how four or more molecules can bond together in a single chain. 3. What does anhydride mean? What would happen to the acetic anhydride if water was present in the reaction mixture? See reactions of acid anhydrides in McMurry, section 21.5 (pg 835). How would this affect the aspirin reaction? See paragraph 2 on page 1 of this handout. 4. Because water is polar, it dissolves ions very well, but not organic compounds. As a result, the solid product dissolves in basic solution and then recrystallizes in acid solution. Use structural formulas to depict balanced acid-base reactions of the solid aspirin product with HCO3-1 and of the dissolved anion with H+. Include all (s) and (aq) phase subscripts. 5. What substance are you “listening” for when NaHCO3 is added in step B1? Provide a balanced decomposition reaction for the HCO3- ion with H+. Include phase subscripts: (aq), (liq), and (g).