Chemistry
advertisement
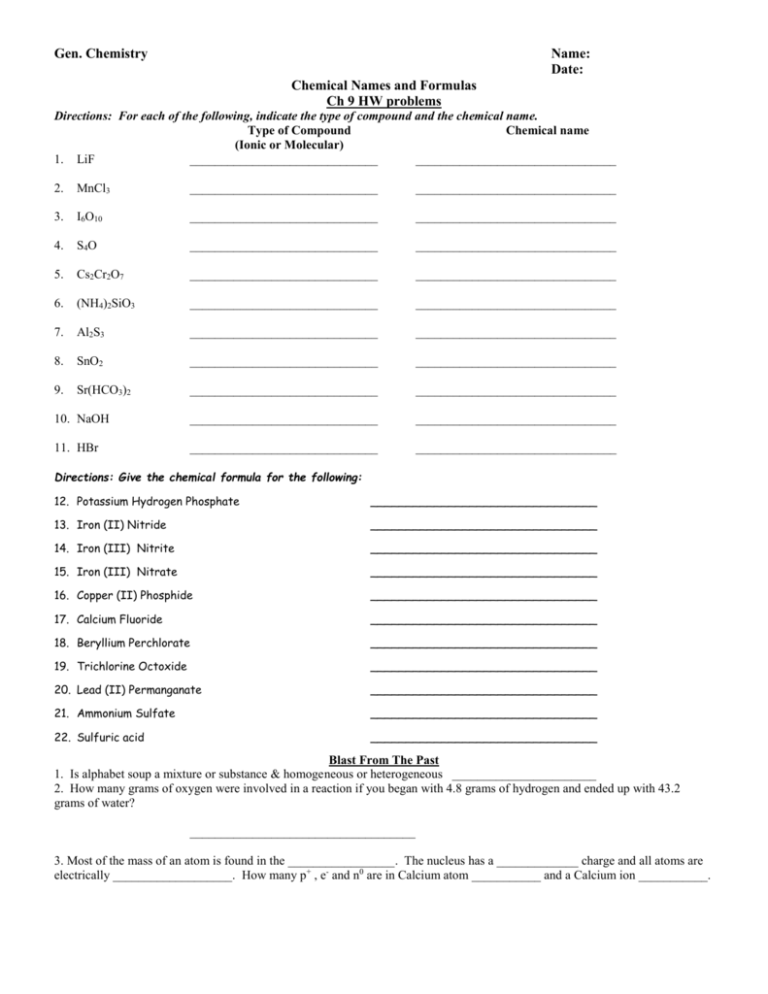
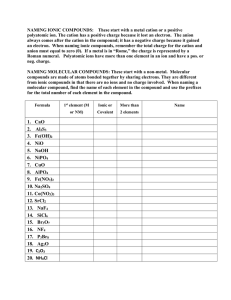
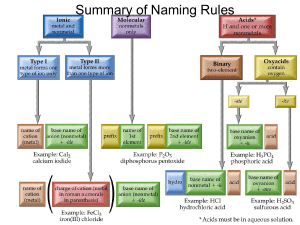
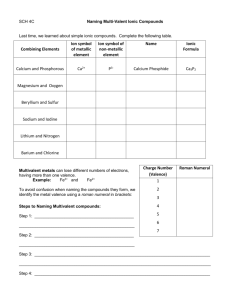
Gen. Chemistry Name: Date: Chemical Names and Formulas Ch 9 HW problems Directions: For each of the following, indicate the type of compound and the chemical name. Type of Compound Chemical name (Ionic or Molecular) 1. LiF ______________________________ ________________________________ 2. MnCl3 ______________________________ ________________________________ 3. I6O10 ______________________________ ________________________________ 4. S4O ______________________________ ________________________________ 5. Cs2Cr2O7 ______________________________ ________________________________ 6. (NH4)2SiO3 ______________________________ ________________________________ 7. Al2S3 ______________________________ ________________________________ 8. SnO2 ______________________________ ________________________________ 9. Sr(HCO3)2 ______________________________ ________________________________ 10. NaOH ______________________________ ________________________________ 11. HBr ______________________________ ________________________________ Directions: Give the chemical formula for the following: 12. Potassium Hydrogen Phosphate ________________________________ 13. Iron (II) Nitride ________________________________ 14. Iron (III) Nitrite ________________________________ 15. Iron (III) Nitrate ________________________________ 16. Copper (II) Phosphide ________________________________ 17. Calcium Fluoride ________________________________ 18. Beryllium Perchlorate ________________________________ 19. Trichlorine Octoxide ________________________________ 20. Lead (II) Permanganate ________________________________ 21. Ammonium Sulfate ________________________________ 22. Sulfuric acid ________________________________ Blast From The Past 1. Is alphabet soup a mixture or substance & homogeneous or heterogeneous _______________________ 2. How many grams of oxygen were involved in a reaction if you began with 4.8 grams of hydrogen and ended up with 43.2 grams of water? ____________________________________ 3. Most of the mass of an atom is found in the _________________. The nucleus has a _____________ charge and all atoms are electrically ___________________. How many p+ , e- and n0 are in Calcium atom ___________ and a Calcium ion ___________. Chemistry Name: Date: Chemical Names and Formulas Ch 9 HW problems Directions: For each of the following indicate the type of compound and write the chemical name. ____________________________________________________________________________________________ Type of Compound Chemical name (Ionic, Molecular, or Acid) ___________________________________________________________________________________________ 1. AlCl3 _____________________________ ______________________________ 2. CuBr2 _____________________________ ______________________________ 3. Cl2O8 _____________________________ ______________________________ 4. Fe(MnO4)2 _____________________________ ______________________________ 5. HF _____________________________ ______________________________ 6. NaH2PO4 _____________________________ ______________________________ 7. SrSO3 ______________________________ _____________________________ 8. Ba(C2H3O2)2 _____________________________ ______________________________ 9. P4O9 _____________________________ ______________________________ 10. H2SO4 _____________________________ ______________________________ 11. NO2 _____________________________ ______________________________ 12. HCl _____________________________ ______________________________ 13. H3PO4 _____________________________ ______________________________ Directions: Write the chemical formula for the following. 14. Lithium Hydrogen Sulfate ______________________________ 15. Chromium (II) Phosphite ______________________________ 16. Chromium (III) Phosphide ______________________________ 17. Chromium (II) Phosphate ______________________________ 18. Mercury (II) Bromide ______________________________ 19. Calcium Iodide ______________________________ 20. Carbon Tetrachloride ______________________________ 21. Nitrous acid ______________________________ 22. Cobalt (III) Oxalate ______________________________ 23. Tin (IV) oxide ______________________________ 24. Sulfur pentachloride ______________________________ Directions: Answer the following questions below. 25. Would you expect the following compounds to be ionic or molecular? NaOH ______________ CO ____________ 26. How many protons and electrons are in each of these ions? Calcium ion ____________ Phosphide ion ____________ Rubidium ion ____________