Polar Water Lab Worksheet: Solubility & Polarity
advertisement

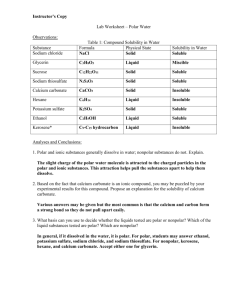
Name _____________________________________ Subject __________________, Period ____ Teacher __________________ Texas High School Date ____________ Lab Worksheet – Polar Water Observations: Substance Sodium chloride Table 1: Compound Solubility in Water Formula Physical State Solubility in Water Glycerin Sucrose Sodium thiosulfate Calcium carbonate Hexane Potassium sulfate Ethanol Kerosene* Analyses and Conclusions: 1. Polar and ionic substances generally dissolve in water; nonpolar substances do not. Explain. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. Based on the fact that calcium carbonate is an ionic compound, you may be puzzled by your experimental results for this compound. Propose an explanation for the solubility of calcium carbonate. _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ 3. What basis can you use to decide whether the liquids tested are polar or nonpolar? Which of the liquid substances tested are polar? Which are nonpolar? _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________