Chemical Reactions Worksheet: Key Concepts
advertisement
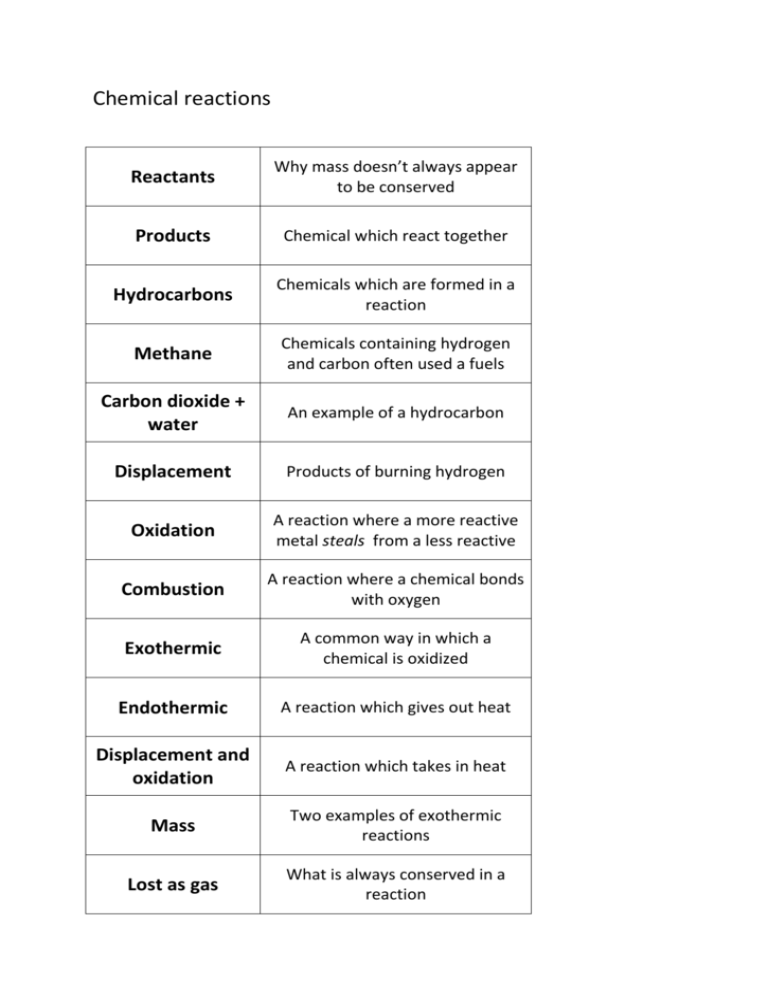
Chemical reactions Reactants Why mass doesn’t always appear to be conserved Products Chemical which react together Hydrocarbons Chemicals which are formed in a reaction Methane Chemicals containing hydrogen and carbon often used a fuels Carbon dioxide + water An example of a hydrocarbon Displacement Products of burning hydrogen Oxidation A reaction where a more reactive metal steals from a less reactive Combustion A reaction where a chemical bonds with oxygen Exothermic A common way in which a chemical is oxidized Endothermic A reaction which gives out heat Displacement and oxidation A reaction which takes in heat Mass Two examples of exothermic reactions Lost as gas What is always conserved in a reaction