The Incredibly Fun & Tasty Atomic Mass of Candinium Lab
advertisement

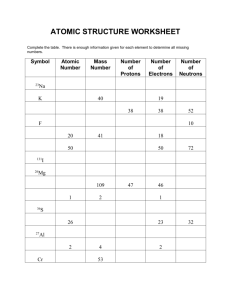
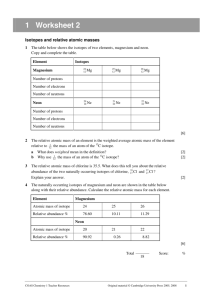
The Incredibly Fun & Tasty Atomic Mass of Candinium Lab (LAB GUIDELINES: 1,2,3,4,6[attach handout],7,9,10,12) Purpose: To develop a strategy for determining the average atomic mass of a fictional element called candinium, which has 3 isotopes: plainium, jellibellium, and fruitium. Introductory Information: Isotopes The atomic mass unit (amu) and the atomic mass of an atom (different than mass number) Average Atomic Mass of an element Procedure: Analyze your individual sample of candinium in order to complete the following data table. Remember: do not eat up your sample until you are sure that you have filled in the table correctly! (Also, you are not allowed to consider the mass of the entire sample in solving this problem, as the sample tends to rapidly oxidize, lose electrons, and explode when all isotopes are placed on a balance together.) Data: (Note: You must create your own data table on your paper!!) Total # of candinium atoms in sample_________ Isotope # of atoms of this isotope present total mass of all the atoms of this isotope Avg. mass of this isotope (show calc.) % abundance of this isotope (show calc.) Plainium Jellibellium Fruitium Calculations: Now, using only the last two columns of your data table, calculate the average atomic mass of candinium. Show your work. Questions: 1. The element magnesium (Mg) has three stable isotopes with the following masses and abundances Isotope Mass (amu) Abundance Magnesium-24 23.985 amu 78.99% Magnesium-25 24.986 amu 10.00% Magnesium-26 25.983 amu 11.01% Calculate the average atomic mass of magnesium from these data. 2. An element is a mixture of two isotopes. One isotope has an atomic mass of 34.969 amu and an abundance of 75.53%. The other isotope has an atomic mass 36.966 amu. Create a data table for this element like the one shown in #1. Calculate the average atomic mass of the element, and then use your periodic table to identify the element. 3. Reflect on all that you have done in this lab activity and briefly explain why the atomic mass reported for each element on the periodic table is not a whole number. Conclusion: Write a conclusion in which you: restate the purpose, summarize your procedure and data recorded, explain the math, and finally give your results. Also, in your conclusion, explain the concept of a weighted average, and how it applies to the determination of the average atomic mass of the elements on the Periodic Table. Remember, here is a great guide for writing your conclusion: 1) Restate the purpose 2) Summarize the procedure and data collection 3) Discuss any important principles / equations used 4) Explain the graphs and/or math 5) Report your results Also, please include any other interesting information you would like to add.