Phenol chemistry - Miller, Jonathan
advertisement
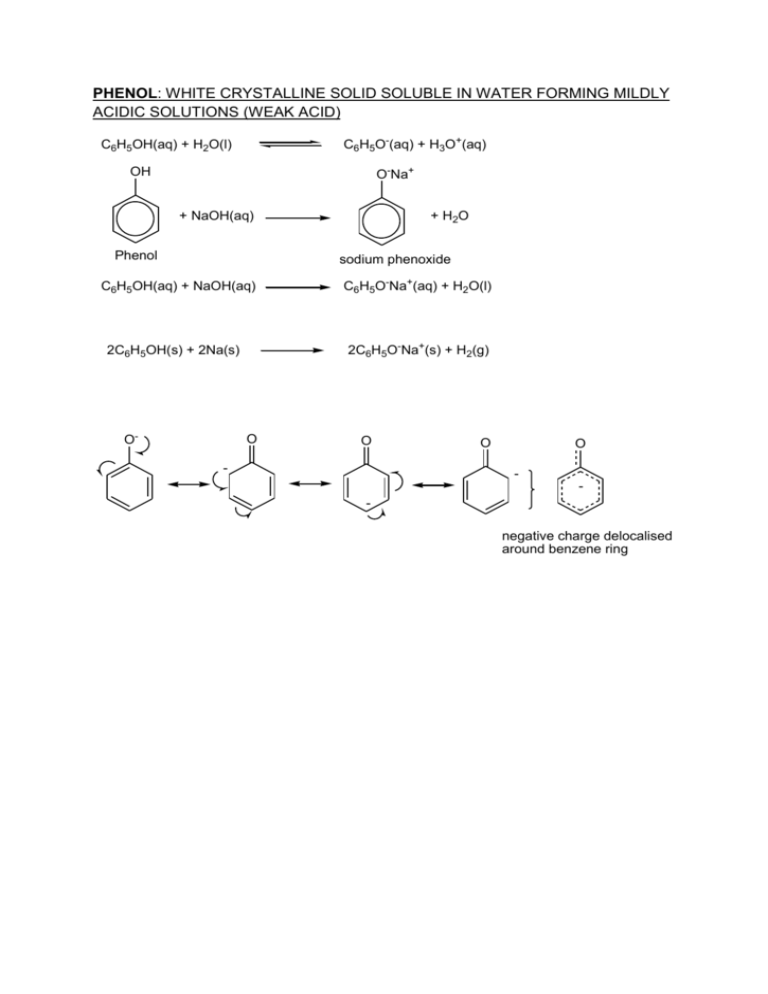
PHENOL: WHITE CRYSTALLINE SOLID SOLUBLE IN WATER FORMING MILDLY ACIDIC SOLUTIONS (WEAK ACID) C6H5O-(aq) + H3O+(aq) C6H5OH(aq) + H2O(l) O-Na+ OH + NaOH(aq) Phenol + H2O sodium phenoxide C6H5OH(aq) + NaOH(aq) 2C6H5O-Na+(s) + H2(g) 2C6H5OH(s) + 2Na(s) O- C6H5O-Na+(aq) + H2O(l) O O - O O - negative charge delocalised around benzene ring interaction of the lone pair on oxygen with delocalised -bonding: stabilises positive charge + OH .. OH OH OH -Br+ + H Br Br + H H Br Br H Br bromine electrophile OH + H OH + Br- + HBr Br Br Phenol is therefore much more reactive than benzene towards electrophilic substitution reactions and becomes trisubstituted (phenol is ortho, meta, paradirecting or net 2,4 directing effect). The neg. charge at C-4 is more stable. OH + + OH + OH - OH - + OH - - lone pair from oxygen interacts with delocalised benzene ring C6H5OH(aq) + 3Br2(aq) C6H2Br3OH(s) + 3HBr(aq) Bromine water can be used and the solution becomes decolourised forming a white precipitate of 2,4,6-tribromophenol; the product has a characteristic anti-septic smell. BEYOND A LEVEL: The image below shows the electron charge density of phenol. The blue colour on the electron charge density image reveal more electron charge (i.e. more negative charge, -) and the red less electron density (+); it therefore shows the preferable attack of the bromine electrophile at the 2,4 and 6 positions (blue colour around the carbon atoms). The oxygen atom is still clearly blue because of its electronegativity and lone pairs despite overlap with the benzene ring and some loss of electron density. The oxygen atom has a negative inductive (-I) and positive mesomeric (+M) effect on the ring carbon atom adjacent to it. Without or less of these effects, it is much harder to functionalise the benzene ring in this manner. The functionalisation here is to substitute a hydrogen atom for another atom/group. The 3D image of phenol was geometrically optimised (semi-empirical AM1) using molecular modelling prior to showing the surfaces. This should wet your appetite for any future higher level studies!