Electron Cloud Activity Key: High School Chemistry
advertisement

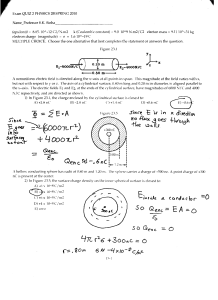
Name:____________KEY__________________Date:_____________________Period:_____ Electron Cloud Activity Key Objective 1. Graph the distribution of electrons around a central nucleus. Introduction The position of an electron in an atom cannot be determined at any given moment. However, the region, or cloud, in which the electron can probably be found is predictable. The shape of these clouds is determined mathematically using the wave-mechanical model of the atom. This lab simulates the probable distribution of electrons around a central atom in a spherical s-orbital. Materials (per lab group) Felt-Tip Marker Construction Paper Procedure 1. Draw a central circle on the construction paper. Draw four equally spaced concentric rings around the central circle. Your drawing should look like a target, filling up the paper. 2. Place your target on the floor. 3. Drop your felt-tip marker from a height of 1 meter onto the target so that it marks the target. Try to aim for the center bull’s eye. Repeat procedure 100 times. 4. Tally the number of marks in each ring of the circle. Record your data in the table. 5. Plot your results on a piece of graph paper. The number of marks should be plotted on the y-axis and the numbered area on the x-axis. Data Table (Example and graph) Number of Marks 29 25 21 16 9 Distribution of Marker Hits 35 30 Number of hits Ring Number 1 (innermost) 2 3 4 5 25 20 15 10 5 0 1 2 3 Ring number 4 5 Questions 1. Which target ring had the highest probability of a hit? Answers may vary—Ring 1 usually has the highest probability 2. How are orbital pictures similar to your target? Answers may vary. The “hit” target illustrates the probability of where a single s-electron might be found in its orbital. Page 1 of 1 © 2004 High School Technology Initiative (HSTI) Educational Materials: The ATOM: Structure