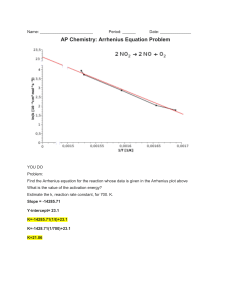
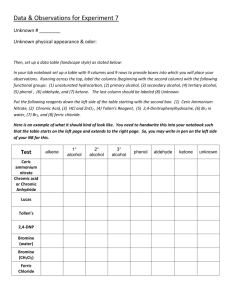
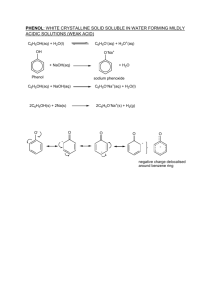
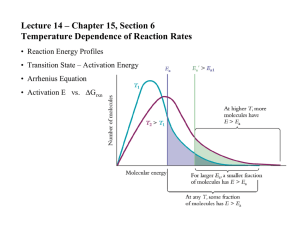
Experiment P04
advertisement

Experiment P04 To determine the activation energy of the reaction between Br- and BrO3- in acidic solution by using the Arrhenius equation Questions: 1. Write an equation for the reaction between bromine and phenol. OH OH Br 3 Br2 Br + + 3 HBr Br 2. What function does the phenol and methyl red play in this experiment? The bromine produced will firstly react with a fixed amount of phenol quickly. Subsequently, it will react with the methyl red indicator and bleach it. 3. Why is it unsatisfactory to measure the reaction rate at high temperature such as 90oC? The time for disappearance of red colour will be too short to be measured and the percentage error arisen will also be large. 4. Arrhenius equation is given as k = A e What do k and Ea represent? k: rate constant. Ea: activation energy. Ea RT