Discuss on the Theory of Substitution Effects
advertisement

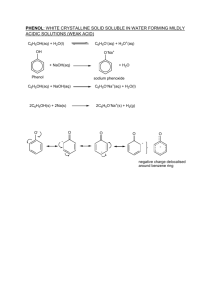
Discuss on the Theory of Substitution Effects Submitted by WWW.ASSIGNMENTPOINT.COM www.AssignmentPoint.com Ring activators are groups that increase the electron density on the benzene ring and thereby make the ring more susceptible to electrophilic aromatic substitution reactions. Ring deactivators decrease the electron density on the benzene ring, thus making the ring less reactive toward electrophilic aromatic substitution reactions. Resonance theory can be used to illustrate these processes. Ring Activation Most ring activators have atoms with unshared electron pairs directly attached to a carbon atom of the benzene ring. For example, the — OH group has two pairs of unshared electrons on the oxygen atom, which will form a bond to a carbon atom of the benzene ring. Thus, the — OH group will be an activating group. The following illustration shows why this group will act as an ortho‐para director. Notice that three of the four resonance structures show a negative charge residing on the positions ortho and para to the — OH group. These electron‐rich positions should attract an electrophile more strongly than the less electron‐rich meta positions do. Therefore, any group that possesses unshared electron pairs on the atom directly attached to a carbon atom of the benzene ring will be an ortho‐para (activating) group. Groups that do not have unshared electron pairs on the atom directly attached to the benzene ring may also www.AssignmentPoint.com supply electrons to the benzene ring. This situation occurs if the atom in a group has weakly bonded π electrons attached to it or if the group has an inductive effect associated with it. The following diagram shows an example of the π electron movement giving ring activation. As with the — OH group example, the ortho and para positions are electron‐rich compared to the meta positions. Thus ortho‐para substitution occurs. Ring Deactivation Groups that withdraw electrons from the ring will deactivate the ring and act as meta directors. Groups capable of doing this usually contain an atom that is directly attached to a carbon atom of the benzene ring and that bears a positive or partially positive charge. A typical example is the nitro group — NO 2. The structure of the nitro group is: www.AssignmentPoint.com Notice that in three of the four resonance structures, a positive charge exists on the ortho and para positions. Thus, the hybrid structure is electron‐poor in these areas, meaning that an electrophile generally attaches to the more electron‐rich meta position. www.AssignmentPoint.com