Chem 3125 - St. Edwards University
advertisement
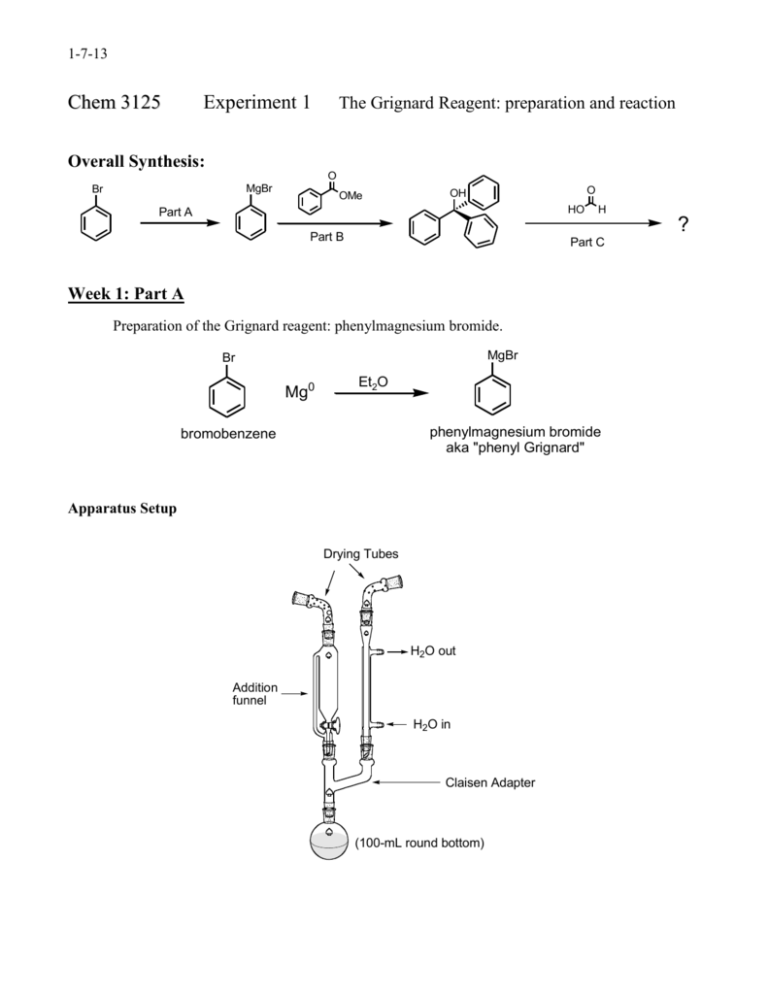
1-7-13 Ch e m 3 1 2 5 Experiment 1 The Grignard Reagent: preparation and reaction Overall Synthesis: O MgBr Br OMe O OH HO Part A H ? Part B Part C Week 1: Part A Preparation of the Grignard reagent: phenylmagnesium bromide. MgBr Br Mg0 Et2O phenylmagnesium bromide aka "phenyl Grignard" bromobenzene Apparatus Setup Drying Tubes H2O out Addition funnel H2O in Claisen Adapter (100-mL round bottom) 1-7-13 Procedure: Special Note: The Claisen adapter, condenser and RB flask must be dried in an oven, preferably overnight. Before retrieving these items from the oven, have the magnesium, addition funnel and drying tubes ready. Add a stirbar and ~ 1.2 g of magnesium turnings to a 100-mL RB flask and assemble the apparatus as shown in the figure above. Add 8 mL of anhydrous ether to the RB to make a suspension of magnesium in ether. To the addition funnel (stopcock closed!!) add 10 mL of anhydrous ether and 4.70 mL of bromobenzene and swirl to homogenize. Ensure that water is running through the condenser and heat the flask with a heating mantle at a setting of 2-3. Add a portion of the bromobenzene/ether solution (~ 20-30 drops) from the addition funnel onto the magnesium turnings. A chalky appearance or color change (brown) is indication that the reaction has started. If the reaction has not started after 10 min, add an additional portion of the bromobenzene/ether solution. If the reaction still hasn’t begun after an additional 10 minutes, add 2 or 3 drops of 1,2dibromoethane to the mixture. This compound reacts very readily with magnesium to form ethylene gas and MgBr2; this removes the unreactive oxide layer on the magnesium, leaving it free to react with the bromobenzene. Once the reaction has started, add an extra 8-mL portion of anhydrous diethyl ether to the reaction mixture through the condenser. This serves to dilute the reaction mixture and to minimize the coupling reaction. The rest of the bromobenzene/ether solution should now be added dropwise to the reaction mixture at a rate that is just fast enough to maintain a gentle reflux. If it is added too fast, the reaction may get out of control, and the yield will also be reduced, owing to increased coupling. If the reaction becomes too vigorous turn off the heating mantle and reduce the rate of addition. This total addition should take about 15 min. If the spontaneous boiling of the mixture becomes too slow, increase the rate of addition slightly and/or turn up the heating mantle At the end of the reaction the solution will normally have a tan to brown color, and most of the magnesium will have disappeared, although residual bits of metal usually remain. Notes: No characterization is required for Part A. Once prepared, the Grignard reagent must be immediately reacted with the ester, the first stopping point being the first asterisk below: 1-7-13 Part B The reaction of phenylmagnesium bromide with methyl benzoate to give triphenylmethanol. Procedure: Dissolve 2.50 mL of methyl benzoate in 8 mL of anhydrous diethyl ether and place this solution in the addition funnel (stopcock closed). Heat the RB flask gently (mantle setting ~1-2), and then begin slow, dropwise addition of the solution of methyl benzoate to the phenylmagnesium bromide solution. This reaction is exothermic; control the rate of reaction by adjustment of the addition rate and/or removing the heating mantle, if necessary. Frequently, a white solid forms during the reaction and is a sign that the reaction is proceeding normally. After the addition is complete and the exothermic reaction has subsided, heat the reaction mixture at gentle reflux for 20 min. Near the end of this time, add H2SO4 (25 mL, 6M) and ~ 20 g of ice to a 250-mL Erlenmeyer flask. After allowing the RB flask to cool to room temperature, pour the reaction mixture into the H2SO4/ice mix with swirling. Stopper the Erlenmeyer and store in the location given by your instructor. Week 2 Add enough methyl tert-butyl ether (MTBE) to dissolve all the solids and transfer the entire mixture to a 125-mL separatory funnel. Shake the funnel vigorously but carefully, venting often to relieve pressure. Remove the aqueous layer and wash the remaining organic first with H2SO4 (25 mL, 3M) and then with deionized water (20 mL). Collect the organic layer and dry over anhydrous magnesium sulfate. Gravity filter into a 100-mL RB flask and distill away most of the solvent. Remove the heating mantle and recrystallize from a mixture of ethanol and water (80/20) or heptane (reread Mohrig book about mixed solvent recrystallizations if you are rusty on recrystallizations). Isolate the product by vacuum filtration, using a cold solution of ethanol and water (~50/50). Allow the solids to dry for at least a day and record the isolated mass. Notes: Triphenylmethanol (MW = 260.3 g/mol; MP = 164.2˚C) Required characterization: melting point, proton NMR (in CDCl3), and IR. Lab report should include analysis of the spectra produced by drawing the structure on the NMR or IR and labeling which peaks go with which functional groups or protons. 1-7-13 Week 3 Part C Formation of an unknown derivative by reaction with HCOOH O OH HO H ? Procedure: In a 50 mL RB flask heat a mixture of 1.00 g of triphenylmethanol in 5 mL of formic acid to reflux for 30 min. Allow the flask to cool to room temperature, isolate the product by vacuum filtration and wash with a little methanol. Recrystallize the solid from ethanol. After isolating the solids by vacuum filtration, allow them to dry for at least a day before weighing. Determine the percent yield of the product based on the molecular weight of the structure you assign. Required characterization: melting point, proton NMR (in CDCl3), and IR spectrum. Lab report should include analysis of the spectra produced by drawing the structure on the NMR or IR and labeling which peaks go with which functional groups or protons.