Grignard Synthesis: Preparation of Benzoic Acid
advertisement

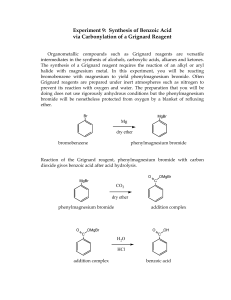
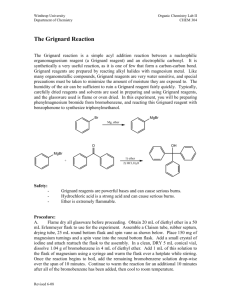
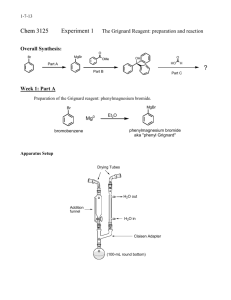
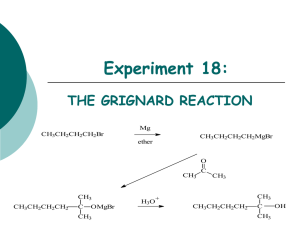
CHEM 310 L Summer 2 Experiment 1 Organic Chemistry Laboratory Name: _________________________________________________________________ Grignard Synthesis: Preparation of Benzoic Acid Br 1. Mg / Et2O 2. CO2 3. H3O+ O OH A Classical method for making new carbon-carbon bonds uses reactants containing magnesium carbon bonds, known as Grignard reagents. Grignard regents react with a variety of classes of organic compounds such as aldehydes, epoxides, esters, acid halides, and nitriles. Compounds containing metal-carbon bonds are called organometallics. Background: Victor Grignard, a French chemist at the turn of the twentieth century discovered that organic halides (R-X, X=Cl, Br, or I) and magnesium react to form organomagnesium halides (R-Mg-X, X=Cl, Br, or I). In 1912 he was awarded the Nobel Prize for this discovery. Grignard reagents may be formed by reacting elemental magnesium with primary, secondary, and tertiary alkyl halides, or aryl and vinyl halides. Assorted Grignard Reagents: Mg Br Br Br MgBr H3C H3C CH 3 Mg Metal CH 3 H3C Br CH 3MgBr Br MgBr Br Br MgBr MgBr 1 CHEM 310 L Summer 2 Experiment 1 Preparation of Benzoic Acid: Phenylmagnesium bromide will be used to produce benzoic acid via reaction with solid carbon dioxide. The resulting salt will be quenched with hydrochloric acid to give the final organic carboxylic acid. You must keep the reaction apparatus and reagents completely dry because water functions as an acid and would kill the Grignard reagent, to produce benzene. All glassware must be clean and dry. Do not wash your glassware at the start of the experiment. When you have finished the experiment, please clean your glassware and place all pieces back in the oven. Reaction: Br bromobenzene Mg Br Mg O C O phenylmagnesium bromide Grignard Reagent O +MgBr O O H+ OH magnesium bromide carboxylate salt benzoic acid Reagent Table: Name Structure Magnesium Mg MW (g/mol) Mp. Bp. Density (˚C) (˚C) (g/ml) 34-40 0.7134 Br bromobenzene Hydrochloric Acid Amount Needed HCl 36.46 (need 6M) Ether Et-O-Et Magnesium Sulfate MgSO4 74.12 Drying Agent 2 CHEM 310 L Summer 2 Procedure: Experiment 1 Making the Grignard Reagent Weigh out 1.0g magnesium ribbon or turnings, scrape the sides of the ribbon or crush the turning to expose a clean metal surface (beneath the outer metal oxide coating). Place the magnesium into a 100ml r.b. flask. In the separatory funnel: ♦ Add 4.5ml dry bromobenzene ♦ Add 15ml dry diethyl ether Add approx. 3ml of this bromobenzene/Et2O solution from the sep funnel to the magnesium As the reaction proceeds, you will observe the development of cloudiness and darkening of the solution. This may take 5-10 minutes. It depends on how fresh the metal is and if it has a clean surface. Once the reaction has started, turn on the cooling water in the condenser and manually mix the solution. Add the remainder of the bromobenzene/ether solution SLOWLY and DROPWISE over 30 minutes. Adding the bromobenzene too rapidly may increase the formation of biphenyl by product. Once all of the bromobenzene/ether solution has been added, rinse the separatory funnel with 3ml ether. Heat the reaction flask while maintaining a reflux for 10 minutes (after all of the bromobenzene/ether and 3ml wash have been added to the reaction vessel). After heating, allow the flask to cool to room temperature and add 20 ml of ether. As quickly as possible, weigh out 10g of dry ice and place it in a clean dry beaker. Cover the beaker with a watch glass to minimize the condensation of water on the surface of the dry ice. The Grignard Reaction Transfer the Grignard solution to the beaker containing dry ice. Swirl and mix the reagent and dry ice. Do not worry if you have an excess amount of dry ice. It will sublime. Neutralize the solution with enough 6M HCl to make the solution acidic. Any extra and unreacted magnesium metal should dissolve in the hydrochloric acid. If any solids are present in the beaker – other than Magnesium, add a little more ether to dissolve the residual solid (this is your product). Decant and/or use a pipet to transfer the upper ether layer to an Erlenmeyer flask. Dry the ether with magnesium or sodium sulfate. 3 CHEM 310 L Summer 2 Experiment 1 Gravity filter the solution into a tared Erlenmeyer flask and boil off the solvent using a hot water bath. Keep your sample until tomorrow. Recrystallize your compound from a minimal amount of water Collect the product on a Büchner funnel and air dry the solid as best as you can Determine the m.p. of your sample the next day. Reaction Apparatus: Drying Tube 100ml round bottom flask Claisen adapter Addition (separatory) funnel Separatory Funnel West Condenser Condenser Drying tube with CaCl2 Claisen Tube Round Bottom Flask Reaction Apparatus 4 CHEM 310 L Summer 2 Experiment 1 Name: Data Sheet Mass of the benzoic acid recovered: ____________________g Theoretical Yield calculation: Theoretical Yield: ____________________% Percent Yield: ______________________% Post Lab Question 1) Draw the product for each reaction: 1. Mg / ether Br O 2. 1. H 3. H3O+ OH 2. MgBr H3C CH3 Post Lab Question 2) Propose an experimental procedure you could utilize to physically separate benzoic acid from a possible byproduct (biphenyl) in a laboratory setting. biphenyl O OH reagent ? benzoic acid in Et2O Discussion (continue on the back of this page): 5