Preparation of a Phenylalcohol by a Grignard Reaction
advertisement

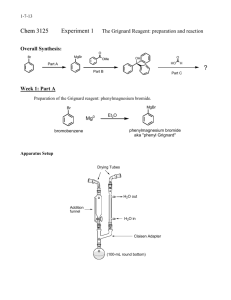
Preparation of a Phenylalcohol by a Grignard Reaction CH344 Bruce A. Hathaway 1 The Reaction OH O Br MgBr Mg ether C O CH2CH3 2-butanone R2 R1 C R2 2. HCl, HOH O H3C 1.R1 C H3C C O CH2CH2CH3 2-pentanone CH3CH2 C CH2CH3 3-pentanone 2 Safety Issues • Bromobenzene causes eye and skin irritation, and inhalation, ingestion, or skin absorption may be harmful. Avoid contact with the liquid and do not breathe its vapors. • Ethyl ether is extremely flammable and may be harmful if inhaled. Do not breathe its vapors and keep it away from flames and hot surfaces. • Magnesium can cause dangerous fires if ignited; keep it away from flames and hot surfaces. 3 The Apparatus • Put some cotton in the drying tube, followed by CaCl2, then by more cotton. • Put the iodine, Mg, and a stir bar in the round-bottomed flask before you assemble the apparatus. • Assemble the apparatus, then remove the thermowell by removing the stirrer; then replace the stirrer. • Start the water flowing slowly through the condenser. Ground-Glass Stopper Drying tube Neoprene Adapter Thermometer Adapter Addition Funnel West Condenser Water Out Claisen Adapter Water In Thermowell To transformer Magnetic Stirrer 4 Reaction of Bromobenzene • Add a crystal of iodine to the round-bottomed flask. • Weigh out about 0.7 - 0.8 grams of magnesium turnings into a weigh boat. • Transfer them to a mortar, and grind them for about a minute, to expose the shiny magnesium. • Weigh 0.6 - 0.7g (record exactly what you use) of clean, dry magnesium turnings and transfer then to the round-bottomed flask. • Add a stir bar to the round-bottomed flask. 5 Reaction of Bromobenzene • Weigh 4.0g of dry bromobenzene into a 25 mL dry Erlenmeyer flask and dissolve it in 5 mL of anhydrous ethyl ether. • Transfer this solution to the addition funnel and stopper it, placing a tiny strip of filter paper between the stopper and the neck of the funnel. • Add the bromobenzene solution all at once to the reaction flask. • Replace the bromobenzene solution in the addition funnel by 8 mL of anhydrous diethyl ether. 6 Reaction of Bromobenzene • Begin stirring the solution, and observe the reaction mixture closely for evidence of a reaction, such as cloudiness and the evolution of bubbles from the magnesium surface. • If the reaction does not begin within 5 minutes or so, consult your instructor. • When the reaction mixture begins to boil quite vigorously without external heating, add the ether drop by drop at a rate just sufficient to keep the reaction mixture boiling. 7 Reaction of Bromobenzene • When all the ether has been added, let the reaction continue until the boiling has nearly stopped, then use a heating mantle or thermowell to heat the reaction mixture under gentle reflux for another 15-20 minutes. • The reflux ring of condensing ether should be in the lower third of the condenser. • If a significant amount of ether evaporates, reducing its volume in the reaction flask, replace it with fresh anhydrous ether. • Do not stop at this point, because the phenylmagnesium bromide solution will not keep for long. • Remove the thermowell, but keep the reaction stirring while you do the next part. 8 Reaction of Phenylmagnesium Bromide • Dissolve 1.4-1.5g of 2-butanone, or 1.7-1.8g of 2-pentanone or 3-pentanone, in 20 ml of anhydrous diethyl ether in a dry Erlenmeyer flask and place it in the addition funnel. • When the reaction mixture has cooled so that the ether is no longer boiling, add this solution drop by drop to the reaction mixture with shaking or magnetic stirring. • The addition rate should be sufficient to keep the ethyl ether boiling gently without external heating. • When the addition is complete, use a heating mantle or thermowell to heat the reaction mixture under gentle reflux for another 30 minutes. • Remove the thermowell to allow the reaction to cool. 9 Reaction of Phenylmagnesium Bromide • After the reaction mixture has cooled to room temperature, add 5 mL of water drop by drop through the addition funnel while shaking or stirring, and then add 25 mL of 5% (1.4 M) hydrochloric acid. (We are using 1M HCl). • Wait for the reaction to subside and continue stirring or shaking until most or all of the white solid has dissolved (some magnesium may remain undissolved). • If any undissolved white solid remains, detach the reaction flask from the apparatus and use a spatula to break up the solid, then shake the capped flask, adding enough diethyl ether or 5% HCl as needed to dissolve all of the solid. • There must be at least 20 mL of ether in the reaction mixture at this time; if necessary, add diethyl ether to replace any ether that evaporated. 10 Separation of the Product • If there is undissolved magnesium present, remove it by gravity filtration through your Hirsch funnel without filter paper, washing the magnesium with a small amount of diethyl ether. • Transfer the reaction mixture to a separatory funnel, and shake gently to mix the layers thoroughly. • Allow the layers to separate, then drain out and discard the lower aqueous layer. 11 Separation of the Product • Carefully wash the ether layer with 15 mL of 5% aqueous sodium bicarbonate. • Then wash the ether layer with 15 mL of saturated aqueous sodium chloride. • Dry the ether solution over anhydrous magnesium sulfate, and filter it into a dry round-bottomed flask. • Evaporate the ether using a rotovap. Put the roundbottomed flask in your drawer until next lab period. • Hopefully, everyone will get at least this far! • We will stop here for the day. 12 Thin Layer Chromatography • Draw a pencil line 1 cm from the bottom of a silica-gel TLC plate. • Make 2 tic marks on the line. • Dissolve a tiny amount of your product in several drops of acetone, and spot it on one of the tic marks. • Finally, spot the TLC standard of biphenyl on the remaining tic mark. 13 Thin Layer Chromatography • Make sure you write down what compound is spotted on each tic-mark in your notebook. • Check the TLC plate under a UV lamp to determine if you spotted enough material. • Put a folded piece of filter paper in a 400 mL beaker, and add 10 mL of 1:19 ethyl acetate:hexane solvent. • Swirl the beaker to wet the filter paper, and then place the TLC plate in the beaker. 14 Thin Layer Chromatography • Cover the beaker with a watch glass, and allow the solvent to rise within 1-2 cm of the top of the plate. • Remove the plate, draw a pencil line at the solvent front, wave the plate in the air to evaporate the solvent off of it, and check the plate under a UV lamp. • Be sure to circle all of the spots. • Pour the solvent into the “Non-Halogenated WaterInsoluble Organic Waste" bottle. • Keep the TLC plate: you will be turning it in with your report. 15 Finishing Up • When you are finished with the hexanes filtrate, pour it into the “Non-Halogenated WaterInsoluble Organic Waste" bottle in the hood. • You will take an IR spectrum of your product. • I will collect your product during the next lab period. • Label a product vial as follows: – – – – Product name Weight Melting point range Your name(s) 16