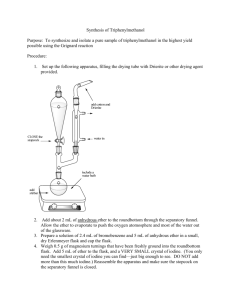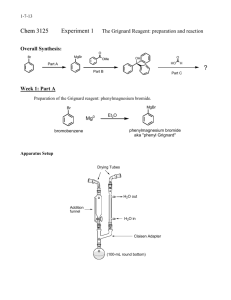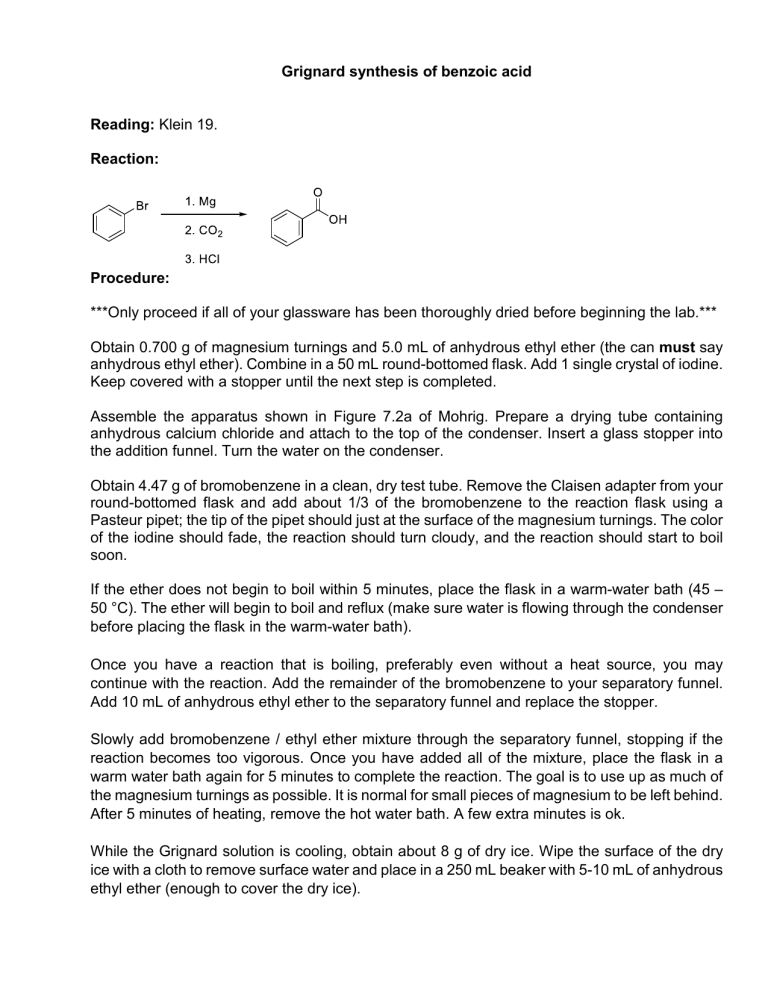
Grignard synthesis of benzoic acid Reading: Klein 19. Reaction: Procedure: ***Only proceed if all of your glassware has been thoroughly dried before beginning the lab.*** Obtain 0.700 g of magnesium turnings and 5.0 mL of anhydrous ethyl ether (the can must say anhydrous ethyl ether). Combine in a 50 mL round-bottomed flask. Add 1 single crystal of iodine. Keep covered with a stopper until the next step is completed. Assemble the apparatus shown in Figure 7.2a of Mohrig. Prepare a drying tube containing anhydrous calcium chloride and attach to the top of the condenser. Insert a glass stopper into the addition funnel. Turn the water on the condenser. Obtain 4.47 g of bromobenzene in a clean, dry test tube. Remove the Claisen adapter from your round-bottomed flask and add about 1/3 of the bromobenzene to the reaction flask using a Pasteur pipet; the tip of the pipet should just at the surface of the magnesium turnings. The color of the iodine should fade, the reaction should turn cloudy, and the reaction should start to boil soon. If the ether does not begin to boil within 5 minutes, place the flask in a warm-water bath (45 – 50 °C). The ether will begin to boil and reflux (make sure water is flowing through the condenser before placing the flask in the warm-water bath). Once you have a reaction that is boiling, preferably even without a heat source, you may continue with the reaction. Add the remainder of the bromobenzene to your separatory funnel. Add 10 mL of anhydrous ethyl ether to the separatory funnel and replace the stopper. Slowly add bromobenzene / ethyl ether mixture through the separatory funnel, stopping if the reaction becomes too vigorous. Once you have added all of the mixture, place the flask in a warm water bath again for 5 minutes to complete the reaction. The goal is to use up as much of the magnesium turnings as possible. It is normal for small pieces of magnesium to be left behind. After 5 minutes of heating, remove the hot water bath. A few extra minutes is ok. While the Grignard solution is cooling, obtain about 8 g of dry ice. Wipe the surface of the dry ice with a cloth to remove surface water and place in a 250 mL beaker with 5-10 mL of anhydrous ethyl ether (enough to cover the dry ice). Disassemble the reflux apparatus and pour the Grignard reagent over the beaker containing the dry ice / ether. Add a stirbar and stir until almost all of the dry ice has sublimed. Add about 30 mL of 1 M HCl in small portions, stirring vigorously. Once you have two layers with no suspended solids, pour the contents of the beaker into your separatory funnel. Extract this solution 3 times with 10 mL of ethyl ether. Use regular ethyl ether (i.e. the bottle should not be labeled anhydrous), combining the ether layers in a 125 mL Erlenmeyer flask. Pour the combined ether layers into the separatory funnel. Wash the ether layer twice with 10 – 15 mL 5 % (w/w) NaOH, draining the aqueous layers into the same 125 mL Erlenmeyer flask. Add 1 M HCl slowly until the solution is acidic to litmus paper. Solid should form at this point. Cool the flask in an ice bath for 10 minutes before filtering through a Buchner funnel. Continue to use the vacuum to draw air through the sample for 5 minutes to dry the sample. Data Obtain an IR spectrum and melting point of the crude product. Name:_____________________ Section:_______ Grignard synthesis of benzoic acid 1. Calculate (15 pts) a) moles of Mg metal used b) moles of bromobenzene used c) which compound is your limiting reagent d) theoretical yield of benzoic acid e) percent yield of benzoic acid 2. Complete the following table with IR peaks that correspond to the functional groups found in your synthesized product. Also, annotate those peaks in your IR printout and turn it in. (10 pts) Wavenumber Functional group 3. In this Grignard synthesis, identify: (5 pts) a. Atoms that have been reduced b. Atoms have been oxidized Type of stretch/bend 4. Explain the role of adding iodine crystal and the consequence if the reaction flask is not dry. (5 pts) 5. Draw the structure of the bromobenzene-derivatives after each step in this synthesis lab. (10 pts)