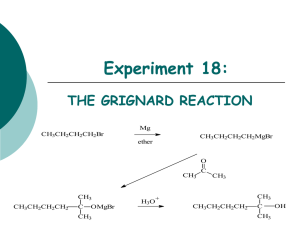
Preparation of a Phenylalcohol by a Grignard Reaction.

Preparation of a Phenylalcohol by a Grignard Reaction
Br
Mg ether
O
MgBr
1.
R
1
C R
2
2. HCl, HOH
R
C
1
OH
R
2
O O O O
R
1
C R
2
Possible
Ketones
H
3
C C CH
2
CH
3
2-butanone
H
3
C C CH
2
CH
2
CH
3
2-pentanone
CH
3
CH
2
C CH
2
CH
3
3-pentanone
Safety Issues
Bromobenzene causes eye and skin irritation, and inhalation, ingestion, or skin absorption may be harmful. Avoid contact with the liquid and do not breathe its vapors.
Ethyl ether is extremely flammable and may be harmful if inhaled. Do not breathe its vapors and keep it away from flames and hot surfaces.
Magnesium can cause dangerous fires if ignited; keep it away from flames and hot surfaces.
The ketones are flammable.
HCl, even 5%, can be corrosive.
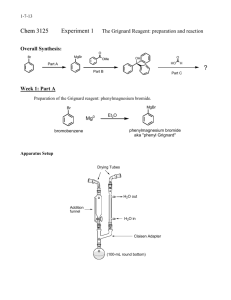
Procedure
Reaction of Bromobenzene.
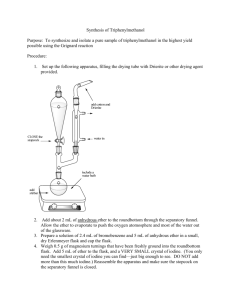
It is essential that all glassware used during this reaction step he clean and scrupulously dried, especially your 100 mL round-bottomed flask. Assemble the apparatus as shown. Add a crystal of iodine to the round-bottomed flask. Weigh out about 0.7 -
0.8 grams of magnesium turnings into a weigh boat. Transfer them to a mortar, and grind them for about a minute, to expose the shiny magnesium. Weigh 0.6 - 0.7g of clean, dry magnesium turnings and transfer then to the round-bottomed flask. Weigh 4.0g of dry bromobenzene into the dried Erlenmeyer flask and dissolve it in 5.0 mL of anhydrous ethyl ether. Then transfer this solution to the separatory-addition funnel and stopper it, placing a tiny strip of filter paper between the stopper and the neck of the funnel. Add the bromobenzene solution all at once to the reaction flask and replace it in the addition funnel by 8 mL of anhydrous diethyl ether. Begin stirring the solution, and observe the reaction mixture closely for evidence of a reaction, such as cloudiness and the evolution of bubbles from the magnesium surface. If the reaction does not begin within 5 minutes or so, consult your instructor. When the reaction mixture begins to boil quite vigorously without external heating, add the ether drop by drop at a rate just sufficient to
1
2 keep the reaction mixture boiling. When all the ether has been added, let the reaction continue until the boiling has nearly stopped, then use a heating mantle or thermowell to heat the reaction mixture under gentle reflux for another 15-20 minutes. The reflux ring of condensing ether should be in the lower third of the condenser. If a significant amount of ether evaporates, reducing its volume in the reaction flask, replace it with fresh anhydrous ether. Do not stop at this point, because the phenylmagnesium bromide solution will not keep for long. Remove the thermowell, but keep the reaction stirring while you do the next part.
Reaction of Phenylmagnesium Bromide.
Dissolve 1.4-1.5g of 2-butanone, or 1.7-1.8g of 2pentanone or 3-pentanone in 20 ml of anhydrous diethyl ether in a dry Erlenmeyer flask, and then transfer the solution to the closed separatory-addition funnel. When the reaction mixture has cooled so that the ether is no longer boiling, add this solution drop by drop to the reaction mixture with shaking or magnetic stirring. The addition rate should be sufficient to keep the ethyl ether boiling gently without external heating. When the addition is complete, use a heating mantle or thermowell to heat the reaction mixture under gentle reflux for another 30 minutes.
After the reaction mixture has cooled to room temperature, add 5.0 mL of water drop by drop through the separatory-addition funnel while shaking or stirring, and then add 25 mL of 5% (1.4
M) hydrochloric acid. Wait for the reaction to subside and continue stirring or shaking until most or all of the white solid has dissolved (some magnesium may remain undissolved). If any undissolved white solid remains, detach the reaction flask from the apparatus and use a spatula to break up the solid, then shake the capped flask, adding enough diethyl ether or 5% HCl as needed to dissolve all of the solid. There should be at least 20 mL of ether in the reaction mixture at this time; if necessary, add diethyl ether to replace any ether that evaporated.
Separation.
If there is undissolved magnesium present, remove it by gravity filtration through your Hirsch funnel without filter paper, washing the magnesium with a small amount of diethyl ether. Transfer the reaction mixture to a separatory funnel, shake gently to mix the layers thoroughly, then drain out and discard the aqueous layer. Carefully wash the ether layer with 15 mL of 5% aqueous sodium bicarbonate. Then wash it with 15 mL of saturated aqueous sodium chloride. Dry the ether solution over anhydrous magnesium sulfate, and filter it through a small plug of cotton into a dry round-bottomed flask. Evaporate the ether using a rotovap.
Check the purity of your solid and the contents of the filtrates by thin-layer chromatography
(TLC). Draw a pencil line 1 cm from the bottom of a silica-gel TLC plate. Make 2 tic marks on the line. Dissolve a tiny amount of your product in several drops of acetone, and spot it on one of the tic marks. Spot TLC standard of biphenyl on the remaining tic mark. Make sure you write down what compound is spotted on each tic-mark in your notebook. Check the TLC plate under a
UV lamp to determine if you spotted enough material. Put a folded piece of filter paper in a 400 mL beaker, and add 10 mL of 1:19 ethyl acetate:hexane solvent. Swirl the beaker to wet the filter paper, and then place the TLC plate in the beaker. Cover the beaker with a watch glass, and allow the solvent to rise within 1-2 cm of the top of the plate. Remove the plate, draw a pencil line at the solvent front, wave the plate in the air to evaporate the solvent off of it, and check the plate under a UV lamp. Be sure to circle all of the spots. Pour the solvent into the "Water-Insoluble
Organic Waste" bottle.
I will collect your product during the next lab period. Turn in the product in a product vial labeled as follows:
Product name
Weight
Melting point range
Your name(s)
You will take an IR spectrum of your product. I will take you down in groups to show you how
NMR spectra are run next week.
3
4
Report Format for Preparation of a Phenylalcohol
A. Title Page:
1.
Descriptive title containing 15-25 words.
2.
Your name.
3.
Your partner’s name, if any.
4.
Course and section numbers.
5.
Dates the experiment was performed.
6.
Tape your TLC plate to the bottom of the page. Clearly label each column so I know what it is.
B. Body of the Report (Start a new page)
1.
Balanced chemical equation, using structures.
2.
Observations (things seen, smelled, etc.), with possible explanations.
3.
Weight of product.
4.
Show the calculations of moles of all reactants from the grams or milliliters you started with.
5.
Show the calculations of theoretical yield in grams, and percent yield. Show your work.
6.
Calculate the R f
values for each of the spots on your TLC plate.
7.
Attach your IR spectrum, and interpret it by labeling significant peaks with possible structural features from the structure of your product.
8.
Attach your proton NMR spectrum, and interpret it by drawing the structure of the product on the spectrum, and drawing arrows from each set of peaks to the hydrogens that produced them. Explain how you assigned each set of peaks, and any coupling patterns, in your report. Identify any peaks for impurities, and tell what impurities made the peaks.
9.
Attach your carbon NMR spectrum, and draw the structure of the product on the spectrum. Identify which peaks were produced by the benzene carbon, the C-O carbon, the CH
2
’s, and the CH
3
’s. Explain your reasoning in your report. Identify any peaks for impurities, and tell what impurities made the peaks.
C. Questions
1.
Write a balanced chemical equation for the formation of biphenyl from bromobenzene and magnesium.
2.
What do you think are the major sources of loss of yield in your experiment? Explain why you think so, citing any evidence from observations you made. What could you have done differently to minimize these losses of yield?
3.
Write an arrow-pushing mechanism to show how your ketone reacted with phenyl magnesium bromide, followed by addition of water, to form your product.
4.
Following the format in Appendix V, and the example for the banana oil lab on page 74, construct a flow diagram for the formation of your phenyl alcohol. Be specific about what impurities are removed by each operation.
Apparatus for Addition with Reflux and Drying Tube
G lass D eoprene A dapter
5






![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-300x300.png)