Introduction Reaction
advertisement

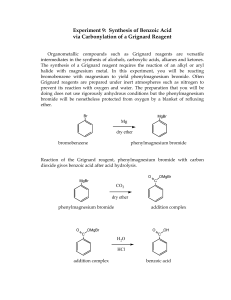
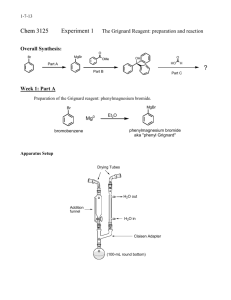
Nucleophilic Acyl Addition Grignard Synthesis of 2-methyl-2-hexanol Introduction The Grignard reaction is one of the most versatile reactions in Organic chemistry. A Grignard “reagent” is and extremely strong base/nucleophile. It is the conjugate base of an Alkane, which are very weak acids. Aldehydes and Ketones undergo Nucleophilic Acyl Addition. The Grignard Reagent is a strong enough base to cause the carbon of the carbonyl group in the Aldehyde or Ketone to behave as an acid. The Grignard can be used to produce alcohols, esters and carboxylic acids. Reaction Step 1 Preparation of the Grignard Reagent Mg Br Ether/Dry MgBr Step 2 Reaction of the Grignard with Acetone O MgBr + OMgBr Step 3 Acidification of the Alcohol Salt H3O+ OMgBr + MgOHBr OH Procedures 1. Equipment Required a. 100 ml round bottom flask b. Claisen adapter c. Separatory funnel d. 25 or 50ml graduated cylinder e. Reflux condenser f. Heating mantle g. Ice bath 2. Chemicals a. Magnesium metal b. Diethyl ether, anhydrous c. 1-butyl bromide d. 10% sodium bicarbonate solution e. Ice 3. Preparation of the Grignard Reagent. a. Weigh 2.43 grams of Mg, 0.1 mole. Add it to the 100 ml round bottom flask. b. Use the graduated cylinder and add 20 ml of ether to the magnesium in the 100 ml round bottom flask. c. Clamp the flask at the neck and allow enough room below to add heat using a heating mantle or cooling, an ice bath. d. Attach the Claisen adapter. e. Add 11.0 ml of 1-bromobutane to the separatory funnel and 20 ml of ether. Attach this to the “arm” that is not directly above the round bottom. f. Attach the condenser directly above the round bottom. The condenser should be cooled. Water in at the bottom and out at the top. g. Add approximately 1/3 of the 1-bromobutane-ether mixture to the round bottom. 4. 5. 6. 7. i. Heat the mixture with the mantle until boiling. Remove the heat source. Continue this cycle until the mixture boils on its own. Have an ice bath ready to moderate the reaction if it becomes too vigorous. h. After the mixture stops reacting spontaneously heat it with a mantle for an additional 15 minutes. Reaction of the Grignard with Acetone a. Add 7.4 ml of acetone, 0.1 mole to the separatory funnel and 10 ml of ether. b. Insert the round bottom flask in an ice bath. c. Add the acetone/ether mixture to the Grignard reagent slowly with cooling. Stir frequently during the addition. Acidification of the salt of the alcohol. a. Add 25 ml of ice water from the previous step to the separatory funnel followed by 10 ml of concentrated sulfuric acid. b. Deliver the acid solution slowly with external cooling to the salt of the alcohol via the attached funnel. After addition, no solids except a small amount of unreacted magnesium metal should remain. Purification and separation of the Alcohol. a. Transfer the mixture to a clean separatory funnel. b. Drain the lower layer aqueous layer into a beaker. c. Drain the ether layer into another small beaker. d. Add the original aqueous layer back to the funnel and extract with two 10 ml portions of ether. e. Add the ether extracts to the original ether layer. f. Return the ether extracts to the sep funnel. i. Wash with two 10 ml portions of 10% sodium bicarbonate. ii. Drain the wash layers and discard. g. Dry the remaining ether layer over CaCl2 until it is clear. Recovery of the 2-methyl-2-hexanol a. Set up a simple distillation to remove the ether, to be saved and collect the alcohol b. Distill the alcohol into a preweighed sample vial. 8. Product Analysis a. Determine the actual yield of the product. b. Measure its refractive index. c. Run the IR. Label the following i. Identify the O-H stretch ii. Identify the C-H stretch iii. Identify Methylene and Methyl stretch iv. Identify the C-O stretch.