Document
advertisement

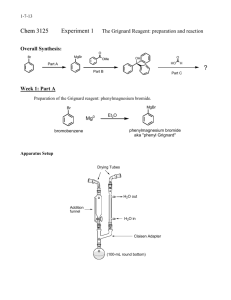
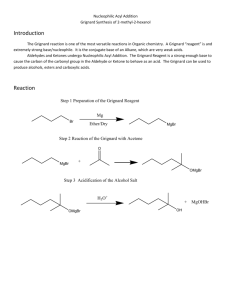
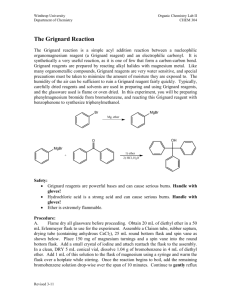
Background: In the early nineteenth century, people wore exclusively natural fibers (e.g. cotton, wool, silk, etc.). The only dyes available at the time were from natural sources, such as fruits and flowers, and most of these lacked light stability and/or water fastness. For centuries, the color purple has been associated with royalty. Tyrian purple, was a rare and expensive dye made from certain mollusks. The extraction process was complicated and the results were variable. In 1856, William Perkin, an eighteen-yearold student at London’s Royal College of Chemistry, created an intense purple dye later called mauveine, from a mixture of aniline, o- and p-methylaniline, and an oxidant. Mauveine (a mixture of several components), was the first of the aniline dyes. Perkin had the foresight to patent his procedure and sent samples of his dye to a textile manufacturer. It dyed silk well (if unevenly), but trials with cotton were not so successful as the necessary mordants (chemicals that fix dyes in or on a substance by forming an insoluble compound) were not yet discovered. Perkin later found that dying silk in a soap bath resulted in even color and that using tannins as one of the mordants allowed the dying of cotton with any shade of color intensity possible. In 1862, Queen Victoria wore a mauve gown to the Royal Exhibition spurring public demand for the new synthetic organic dyes. The dye industry was the first commercial use of synthetic chemicals (not found in nature). A few years later, the triphenylmethane dyes were discovered. Another class of dyes, the azo dyes, were developed in Germany and led to the discovery of the first antibiotics, the sulfa drugs. What property makes a molecule colored (absorb in the visible region of the electromagnetic spectrum)? Chromophore – the extended pi system of a molecule over which pi electrons can be delocalized and which is responsible for the absorption of light The longer the extended pi system, the higher the wavelength of light that is absorbed. Representative dyes based on the triphenylmethyl (trityl) carbocation. Why is the reduced form of malachite green colorless? The extensive delocalization of the pi electrons is responsible for the absorbance of the triphenylmethyl cations in the visible region. Steric hinderance between the ortho hydrogens makes it impossible for the three phenyl rings to be coplanar. Instead the triphenylmethyl carbocation assumes a “propeller” conformation with the phenyl rings pitched at a 32° angle. The triphenylmethyl cation can be prepared by reaction of a Grignard reagent with benzophenone to form triphenylmethyl alcohol. Treatment with fluoboric acid results in the trityl carbocation. Safety Notes for Expt. 30 – Synthesis of Triphenylmethanol and the Trityl Carbocation Physical Properties of the Reactants and Reagents in Expt. 30 IR Spectra of bromobenzene and benzophenone If you do not do a prelab for this experiment, you will be asked to leave and receive a zero for this lab. Step 1: Preparation of the Grignard reagent. Any trace of water (including in the air) will react with the Grignard reagent. What does it form? Setup of the round-bottom flask, Claisen adapter, West condenser and addition funnel A drying tube is added to the top of the condenser Caution: Use diethyl ether only in the hood. It is extremely volatile and flammable. It can form vapor trails across a room and if ignited, will flash back to its source. Be careful not to have ether come in contact with a hot surface. Note: There will not be any oven or flame drying as described in the book. Set up your apparatus (must be dry) for the preparation of the Grignard reagent (flask, Claisen adapter, condenser, addition funnel); your TA will come around and inspect it before giving you 60-70 mL of anhydrous ether. Weigh 22.0 mmol of dry magnesium turnings into your round-bottom flask. Weigh 22.0 mmol of bromobenzene into a dry Erlenmeyer flask and dissolve it in 5 mL of anhydrous ether. Transfer this solution to the addition funnel and add to the Mg turnings in the reaction flask. Carefully rub the Mg turnings with the flat end of your glass stir rod. The reaction will have started when you see tiny bubbles forming on the Mg surface and a cloudiness forms in the solution. If the reaction doesn’t start after 510 minutes, you may add a grain of iodine (I2) to your solution. When the solution boils on its own without heating, start adding ~30 mL of anhydrous ether dropwise with the addition funnel. After the addition (boiling is nearly stopped), maintain a gentle reflux for another 15 minutes using a water bath. Add more ether if necessary to maintain the volume. Dissolve 20.0 mmol of benzophenone in 10 mL anhydrous ether in a dry Erlenmeyer flask and add to the addition funnel. Add a magnetic stir bar to your reaction flask and add the Grignard reagent dropwise to the solution when it is no longer boiling. Add the benzophenone solution rapidly enough to keep the solution boiling without external heating. After the addition, gently reflux the reaction mixture for another 15 minutes and then allow to cool to room temperature. Add 5 mL of water dropwise through the addition funnel and then 15 mL of 5% HCl. Use a spatula to break up any chunks of undissolved solid and add ether and/or 5% HCl to dissolve the solids. If any undissolved Mg remains, remove it by gravity filtration. Transfer the ether/water mixture to a separatory funnel, shake carefully (venting frequently), then let the layers settle before draining and discarding the aqueous layer. Carefully wash the ether layer with 5% sodium bicarbonate. What are the bubbles that form? Wash the ether layer once with sat. sodium chloride, before drying with sodium or magnesium sulfate. Filter the drying agent and evaporate the solvent under vacuum. Add ~10 mL of petroleum ether (PE) to the solid residue and use a spatula or glass stir rod to triturate the solid for 2-3 minutes. Collect the solid by vacuum filtration and wash with fresh PE. Let the solid air-dry on the filter. What is the purpose of this trituration? Recrystallize the crude triphenylmethanol with a 2:1 mixture of ligroin (high bp hexanes) and ethanol. This procedure can be done the following week, if necessary. Use a minimal amount of solvent and let the solution sit undisturbed for at least 30 minutes as the crystals form slowly. Filter, dry and weigh the crystalline solid. During the second part of the experiment, triphenylmethyl alcohol will be converted into triphenylmethyl fluoborate by mixing 1.00 g of the alcohol with 7.9 mL of acetic anhydride in a dry Erlenmeyer flask. Add 1.0 mL of 48% fluoboric acid (HBF 4) and swirl to dissolve all solids. Let sit for 15 minutes, stopper the flask and cool in an ice bath. Collect the precipitated solid by vacuum filtration, rinse with cold anhydrous ether and let it air-dry on the filter. Weigh and calculate your yield. What is the purpose of the acetic anhydride?