Ideal Gas Law Notes
advertisement

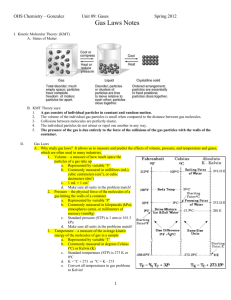
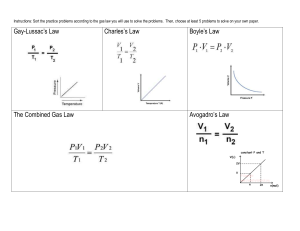
Ideal Gas Law The "Basic" gas laws gave us the following relationships: Avogadro's Law: V α n Boyle's Law: V α 1/P Charles' Law: V α T These relationships can be combined into a single expression that relates volume to the number of moles present, the temperature and the pressure of the gases. PVα nT As with all proportionalities, the proportional sign can be replaced by an equal sign if a constant is added. Because the constant applies to all gases, it is called the Universal Gas Constant and is given the letter "R". PV = R nT This is known as the Ideal Gas Law. This equation is in your reference table. The value of R is there too, but I’d like to show you where this value comes from. What are the pressure and temp of a gas at STP? 1atm (or 101.3 kPa) and 273K What do we know about the volume of a gas at STP? 1mol = 22.4L So we know values for every variable in the equation, except for R – we can solve for R. Put these numbers in and tell me what R is, including units. If units of atm are used for pressure, liters for volume and Kelvin for temperature, R = 0.0821 L·atm·K-1·mol-1. If we use kPa, R = 8.31 L·kPa·K-1·mol-1. When you are using the ideal gas equation you have to pay attention to what units you are using and select the right value for R. Example: How many moles of a gas would occupy 12 L at a pressure of 100 kPa and a temperature of 307 K? We have kPa for pressure, so we can use the 8.31 value for R, and T is already in Kelvin PV = nRT (100 kPa)(12 L) = n (8.31 L∙ kPa/mol∙ K)(307 K) n = 0.47 moles The Ideal Gas Law is used in most problems that involve a chemical reaction and when the number of moles of gas is involved. Example: A sample of dry gas weighing 4.37 grams is found to occupy 2.850 L at 22.0°C and 0.9737 atm. How many moles of the gas are present? P = 0.9737 atm, V = 2.850 L, T = 22°C 295 K, so we can use R = 0.08206 L atm / mol K PV = nRT (0.9737 atm) (2.850 L) = (n) (0.08206 L atm / mol K) (295 K) n = 0.115 moles What is the molar mass of the gas? This is a very common use of this law and the odds are very good you will see this type of question on a test. The key is to remember the units on molar mass: grams per mole. We know from the problem statement that 4.3548 grams of the gas is involved and we also know how many moles that is from our calculation: 0.115 mol. So all we have to do is divide the grams of gas by how many moles it is: 4.37 g ÷ 0.115 mol = 38 g/mol Can you tell me what gas this is? It must be fluorine because the atomic mass of fluorine is 19, but fluorine is diatomic, and 2*19 = 38.