Instructions: Sort the practice problems according to the gas law you
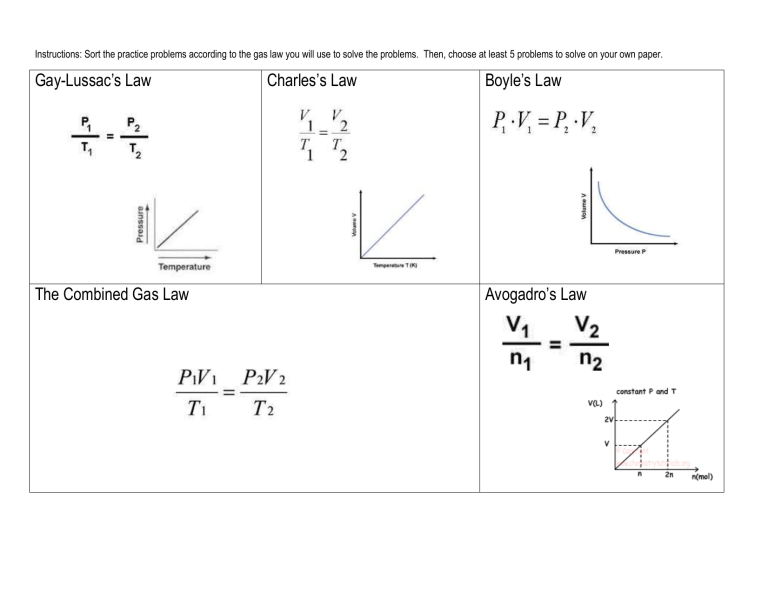
Instructions: Sort the practice problems according to the gas law you will use to solve the problems. Then, choose at least 5 problems to solve on your own paper.
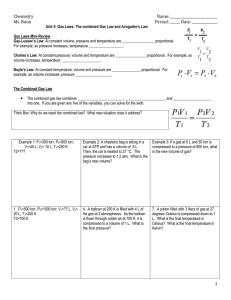
Gay-Lussac’s Law Charles’s Law Boyle’s Law
Avogadro’s Law The Combined Gas Law
1. P1 = 90 torr, T1 = 273 K, P2 = ??, T2 =
2730 K
2. The pressure in a bottle of soda is 505 kPa at 293K. What is the new pressure if someone warms the sealed bottle to
338K?
3. V1 = 50 L, T1 = ??,
V2 = 5 L, T2= 150 K
4. V1 = 75 mL, P1 = 760 mm Hg,
V2 = ??, P2 = 76,000 mm Hg
5. P
V
2
1
=500 torr, P
= 200 L, T
1 n2 = ___ mol
2
=1000 torr, V
1
=200 K, T2=100 K
=?? L,
6. V1 = 10 L, n1 = 50 mol, V2 = 100 L ,
7. At 273K, the volume of a sample of nitrogen is 30 L. What will the temperature be if the volume increases to 300 L?
13. At 500K, a 1000 mL sample is at standard pressure. If the pressure is held constant and the temperature decreases to 5K, what is the new volume in mL?
8. If 2.5 L of a gas at 110.0 kPa is expanded to 4.0 L at constant temperature what will happen to the pressure? What will be the new value of pressure?
14. A balloon with a volume of 15.5 L is inflated in a room at 20.0
° C and then taken outside where the temperature is
7.0
°
C. What is the new volume of the balloon? (Convert to Kelvin first!)
9. A 160 L sample of helium has a temperature of 400K. If the temperature decreases to 100K, what will the new volume be?
19. A 1.0 L volume of gas at 300K exerts a pressure of 760 torr. What will the pressure be at a temperature of 100K?
20. If a gas at 873K exerts a pressure of
1515 mm Hg, at what temperature will the gas exert 151.5 mm Hg of pressure?
15. If 650 mL of hydrogen is stored in a cylinder with a moveable piston at 225 kPa and the pressure is increased to 450 kPa at constant temperature, what is the new volume?
21. A sample of gas in a syringe has a volume of 9.66 mL at a pressure of 64.4 kPa. The plunger is depressed until the pressure is 94.6 kPa. What is the new volume, assuming constant temperature?
10. A sample of argon gas has a volume of 360 mL at standard temperature. What is the volume of the gas if the temperature increases to 300K?
11. 30 mL of gas at STP is heated to 50 degrees Celcius and the pressure is changed to 0.5 atm. Now, what is the volume of the gas?
12. A very large balloon holds 2240 L of helium gas at STP. If 50 moles of helium are released from the balloon, how many moles of helium remain? What is the new volume of helium?
16. A sample of gas occupies 100 L at
3.00 atm. The pressure is decreased to
760 mm Hg. What is the new volume?
17. 2 Liters of a gas at STP is expanded to 4 Liters and 300 K. What is the final pressure?
18. 32 mol of hydrogen gas has a volume of 300 L. If temperature and pressure are held constant, how many moles are in 900
L of hydrogen?
22. A balloon at 200 K is filled with 4 L of
He gas at 2 atmospheres. As the balloon is flown through colder air at 100 K, it is compressed to a volume of 1 L. What is the final pressure?
23. A 1400 mL sample of fluorine gas contains 0.7 mol fluorine. At constant temperature and pressure, what is the volume of 4.9 mol fluorine?
24. What volume of carbon dioxide contains half the number of molecules as
20.0 mL of oxygen at the same conditions?