Quiz 7 – Chemical Reactions and Calculations Due July 28th by 11
advertisement

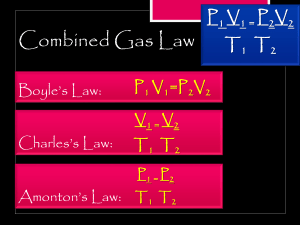
Quiz 7 – Chemical Reactions and Calculations Answers should be submitted using this link. You are encouraged to upload your work for consideration of partial credit. Due July 28th by 11:59 PM Note on the submission form, scientific notation is shown in the form 1.23e-6. This is equivalent to 1.23 x 10-6 1. Please convert each of the following: a. 784 Torr = ________ mmHg b. 0.784 Torr = __________ Pa c. 346 kPa = __________ atm. 2. A sample of gas occupies 4.5 L at 35 ºC and a pressure of 2.6 atm. Determine each of the following: a. Calculate the number of moles of gas in this container. b. Calculate the volume if the pressure changes to 8.4 atm without changing the temperature or moles. c. Calculate the pressure if the temperature increase to 75 ºC without changing the volume or moles. 3. Determine the volume that a 0.625 mole sample of N2 gas at STP occupies. 4. 25 g of solid carbon and 5.0 L of O2 at STP reacts according to the equation: C (s) + O2 (g) CO2 (g) What volume of CO2 is produced if the pressure and temperature do not change? 5. If 3.86 liters of gaseous pentane (C5H12) is mixed with excess oxygen (meaning that O2 is not the limiting reactant) and combusted at STP, determine the volume of CO2 (g) that is produced. Hint: you should start this problem by determining a balanced chemical reaction.