ALKANES, CYCLOALKANES AND ALKENES
advertisement
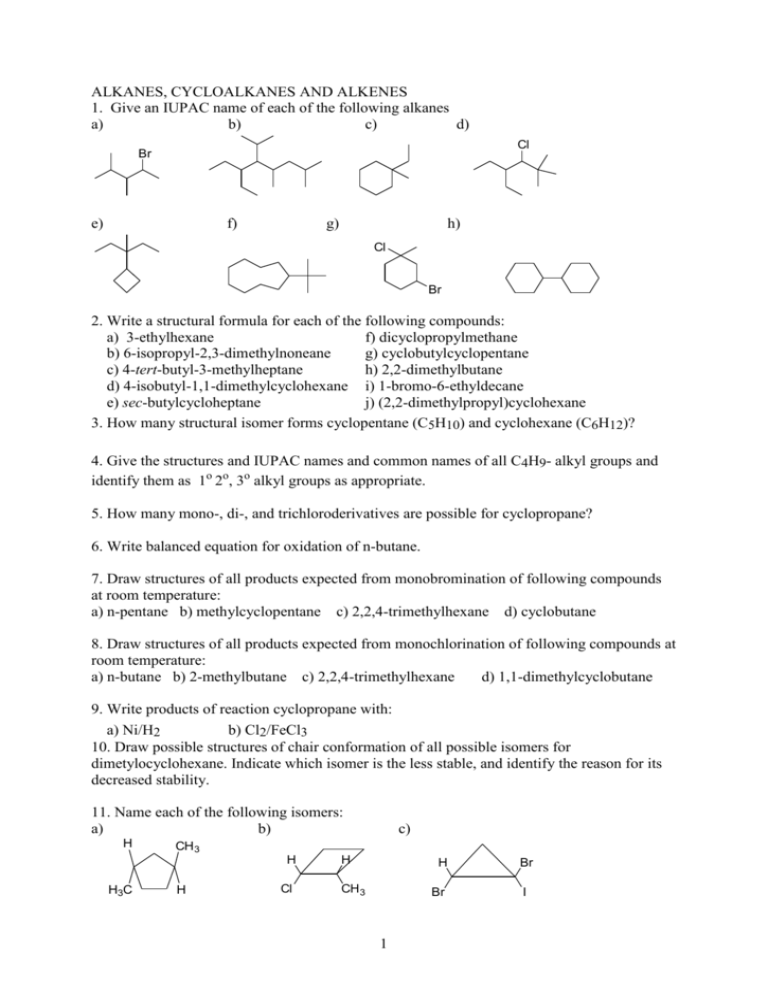
ALKANES, CYCLOALKANES AND ALKENES 1. Give an IUPAC name of each of the following alkanes a) b) c) d) Cl Br e) f) g) h) Cl Br 2. Write a structural formula for each of the following compounds: a) 3-ethylhexane f) dicyclopropylmethane b) 6-isopropyl-2,3-dimethylnoneane g) cyclobutylcyclopentane c) 4-tert-butyl-3-methylheptane h) 2,2-dimethylbutane d) 4-isobutyl-1,1-dimethylcyclohexane i) 1-bromo-6-ethyldecane e) sec-butylcycloheptane j) (2,2-dimethylpropyl)cyclohexane 3. How many structural isomer forms cyclopentane (C5H10) and cyclohexane (C6H12)? Give the structures and IUPAC names and common names of all C4H9- alkyl groups and identify them as 1o 2o, 3o alkyl groups as appropriate. 5. How many mono-, di-, and trichloroderivatives are possible for cyclopropane? 6. Write balanced equation for oxidation of n-butane. 7. Draw structures of all products expected from monobromination of following compounds at room temperature: a) n-pentane b) methylcyclopentane c) 2,2,4-trimethylhexane d) cyclobutane 8. Draw structures of all products expected from monochlorination of following compounds at room temperature: a) n-butane b) 2-methylbutane c) 2,2,4-trimethylhexane d) 1,1-dimethylcyclobutane 9. Write products of reaction cyclopropane with: a) Ni/H2 b) Cl2/FeCl3 10. Draw possible structures of chair conformation of all possible isomers for dimetylocyclohexane. Indicate which isomer is the less stable, and identify the reason for its decreased stability. 11. Name each of the following isomers: a) b) H CH3 H H3C c) H Cl H H CH 3 Br 1 Br I 12. Identify the more stable stereoisomer in each of the following pairs and give the reason for your choice: a) cis- or trans-1-isopropyl-2-methylcyclohexane b) cis- or trans-1-isopropyl-3-methylcyclohexane c) cis- or trans-1-isopropyl-4-methylcyclohexane d) H3C CH3 H3C CH3 CH3 H3C CH3 H3C CH3 CH3 CH3 e) CH3 13. Draw structural formulas of: a) 1,1-dibromo-4-methylcyclohexane b) trans-2-chloro-1-methylcyclobutane 14. Give the structural formula of: 2,3-dimethyl-2-butene 3-bromo-2-methylpropene (Z)-2-methyl-3-heptene (E)-2-chloro-2-butene 1,4-pentadiene (Z)-1,3-pentadiene (E)-2-butene-1-ol 4-methylcyclopentene 15. Name each of the following using systematic IUPAC nomenclature: a) b) c) d) e) f) Cl Br H H Br Cl Br Cl g) (CH3)2C=C(CH3)2 h) (CH3)3CCH=CH2 i) (CH3)2C=CHCH2CH2CH3 j) CH2=CHCH2CH(CH3)2 16. Provide the structural formula of: a) 2,3-dibromo-2-butene b) (E)-1,3-pentadiene c) (Z)-2-chloro-2-butene d) 4-methylcyclohexene e) vinyl bromide f) allyl chloride g) tert-butyl bromide h) neopentyl chloride Br 17. Give structures of alkenes expected from dehydrohalogenation by strong base of: a) 1-chlorobutane b) 2-chloro-2-methylbutane c) 2-bromopentane d) 1-bromo-1-methylcyclohexane e) 1-bromo-2-methylcyclohexane f) 2-bromo-2,3-dimethylbutane Zajcew rule: in dehydrohalogenation the preferred product is the alkene that has the greater number of alkyl groups attached to the doubly-bonded carbon atoms 2 18. Determine the configuration of the following alkenes (Z or E): a) b) Br CH 2OH H 3C I F c) CH 2OH CH 3 d) H3C H CH 2Br H CH 3 CH 3CH 2 CH 3 19. Write the structure of the principal organic product formed in the reactions of styrene (phenylethene) with each of the following: a) HBr b) HCl / peroxides c) H2SO4 / H2O d) 1) BH3, 2) H2O2/ NaOH e) H2 / Pt 20. Write the structures of products forming in the reaction of isobutylene with: a) H2O / H+ b) Cl2 / H2O c) H2SO4 (dimerization) d) 1) (CH3COO)2Hg / H2O 2) NaBH4 e) KMnO4 / H2O f) 1) O3 2) Zn, H2O (ozonolysis) 21. Using cross formulas decide which of the following compounds are chiral, draw models of their enantiomers a) 1-bromobutane b) 2-bromobutane c) 2,3-dibromobutane d) 2,2 dobromobutane 22. Draw and specify as R and S the enantiomers of: a) 2-bromohexane b) 3-chloroheptane c) 3-chloro-1-butene d) 3-bromo-4-methyl-1-pentene e) 1-bromo-1-chloroethane f) 1-bromo-1,2-dichloropropane g) tartaric acid h) trans-1-bromo-2-chlorocyclopentane 3









