Sp04
advertisement

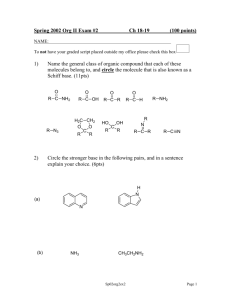
Spring 2004 Org II E2 Ch 18-19 100 points NAME: If you do not wish to have your script placed outside my office, then please check this box 1) Name the general class of organic molecules that each of these molecules belong to, and circle the most reactive molecule with respect to undergoing nucleophilic attack. (7pts) O R C R CH2 R C R N-R R C H O R C Cl 2) Draw a Lewis structure including lone pairs for a molecule of the following general classes: (10.5pts) Alkyl Diazonium Salt Ketone hydrate Isocyanate Sp04org2e2 Page 1 3) Circle the stronger base in the following pairs, and in a sentence explain your choice. (10pts) (a) CH3-NH2 NH3 O HN O HN CH3 CH3 (b) (c) NH3 + NH4 H3C NH (d) CH3 N (e) NH3 H2O Sp04org2e2 Page 2 4) Name the following compounds in IUPAC acceptable terms. (12pts) O O F Br NH2 O C H 5) Explain why in both pairs, the left hand molecule is more reactive toward nucleophilic attack. (10pts) H CH2F H O CH3 O Ph Ph Ph Ph O Ph Ph Ph O Ph Sp04org2e2 Page 3 6) The reaction of 2-butanone with NaBH4 (sodium borohydride) followed by addition of acid yields a chiral product. (i) Draw the product(s) (2pts) (ii) what class of organic compound is the product? (2pts) (iii) what is the name of the general class of this reaction? (2pts) (iv) is the product mixture optically active or racemic? Explain your choice. (4pts) (v) Draw the mechanism for this reaction (8pts) Sp04org2e2 Page 4 7) Give the products formed in five of the following reactions. (15pts) O (a) 1) PhCH2MgBr H3C C CH3 NH2 2) H3O+ excess CH3CH2CH2-Br (b) (c) (d) NH2 excess O H3C C H O Ph C Cl 1)Ph3P, CH3CH2-Br 2) BuLi 3) warm NH2 1) excess CH3I (e) 2) Ag2O, H2O, heat KCN (f) OTos A Sp04org2e2 LiAlH4, H2O Page 5 8) Give reagents for the following transformations. (9pts) O OH OH O O O H H OH OH OH 9) Write the mechanism for the acid catalyzed hydration of propanone (acetone) (8.5pts) *Bonus question* (up to 3pts) Write the mechanism for the reaction of aniline with nitrous acid to generate a diazonium salt. Sp04org2e2 Page 6