Aldehydes/Ketones
advertisement

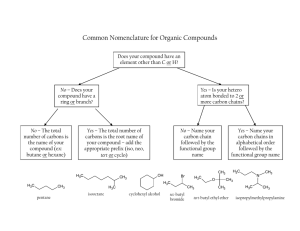
CHM 112 1. SPRING 2007 WORKSHEET FOR ALDEHYDES AND KETONES Write the IUPAC name for each of the following compounds: H2 C H3C H2 C CH CH 3 CH3 C CH3 C H CH O H2 C O CH 3 CH 3 H 2 C C H2 C C H2 CH3 C H2 CH 3 O H2 C C H2 2. H3C H2 C O C O C H Write the full or condensed structural formula for each of the following compounds: 2-ethyl cyclopentanone 4-phenyl-2-octanone 3. H2 C 2-chloro-3, 5-dimethyl heptanal benzaldehyde 3-hydroxy-butanal a. Aldehydes, ketones, and alcohols all have oxygen on their structures. Explain why the boiling points of alcohols are higher than the boiling points of corresponding (about same molar mass) carbonyls. b. Why are aldehydes and ketones more soluble in water than corresponding ethers? 4. Complete the following reactions with the most appropriate reactant(s) or product(s). H2 C K2Cr2O7 H3C H2 C C CH 3 C H2 O H2 C Cu2+ H2 C H3C CH 3 C C H2 O KMnO4 OH CH 3 CH3 H2 C H3C Cu2+ O C C H2 CH3 C H CH3 H2 C H3C [H] O C C H2 CH3 C H CH3 [H] H2 C H3C O C C H2 CH3 C H