electrochemistry review
advertisement

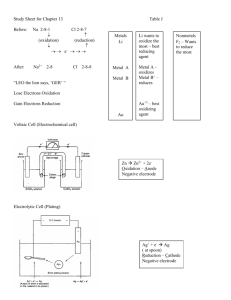
ELECTROCHEMISTRY REVIEW (Chapter 17) Electrochemistry is best defined as the study of the interchange of chemical and electrical energy. GALVANIC / VOLTAIC / ELECTROCHEMICAL CELLS. The above named cell will #1. Convert chemical energy into electrical energy. #2. Be spontaneous going from LEFT to RIGHT which means (a) K > 1 (b) Eo > 0 (c) Go < 0 #3. Electrons will flow from the ANODE to the CATHODE. #4. A salt bridge (filled with a STRONG electrolyte ( such as KNO3) – or porous cup – is needed to prevent a build up of positive charge at the anode and negative charge at the cathode. The salt bridge – porous cup – maintains a balance of charge and provides for ION MIGRATION. #5. Ion migration (a) Anions migrate to the ANODE (b) Cations migrate to the CATHODE. #6. Oxidation occurs at the ANODE. #7. Reduction occurs at the CATHODE. #8. The overall cell reaction is the sum of the ½ reaction at the anode + ½ reaction at the cathode. #9. The overall cell potential is found by Eocell = Eoox + Eored REDOX REACTIONS The overall cell reaction in all cells is a REDOX reaction (oxidation – reduction). This means a transfer of electrons occur – which account for a current. OXIDATION – loss of electrons – the element’s oxidation state becomes more positive – occurs at ANODE. Fe2+ Fe3+ + eIn the above ½ reaction, ferrous ions are OXIDIZED to ferric ions. Ferrous ions are considered the REDUCING AGENT (the donor of electrons). REDUCTION – gain of electrons – the element’s oxidation state becomes more negative – occurs at CATHODE 6e- + 14 H+ + Cr2O72- 2Cr3+ + 7 H2O In the above ½ reaction CHROMIUM IS REDUCED – but dichromate ion is the OXIDIZING AGENT (the acceptor of electrons) BALANCING REDOX REACTIONS (Know how to balance in an acidic and basic solution – refer to Ch 4 notes/review) CELL POTENTIAL The “pull” or DRIVING FORCE on the electrons is called the CELL POTENTIAL (E ocell) or the ELECTROMOTIVE FORCE. The unit of cell potential is the VOLT which is defined as 1 joule of work per coulomb of charge transferred. 1 V = J / c The cell potential is measured with a device called a VOLTMETER. STANDARD REDUCTION POTENTIALS. The ½ reaction that is considered the STANDARD REDUCTION ½ REACTION IS: 2H+ + 2e- H2 Eored = 0.00 volts This ½ reaction is ARBITRARILY ASSIGNED A REDUCTION POTENTIAL OF ZERO!!!! ALL OTHER ½ REACTION REDUCTION POTENTIALS ARE EXPERIMENTALLY DETERMINED BASED ON THIS ½ REACTION. You were given a STANDARD REDUCTION POTENTIAL TABLE for the reduction ½ reactions for many elements, compounds and ions. All of the reduction potentials on that table were determined at 298K and 1 atm (standard conditions). THE MORE POSITIVE THE STANDARD REDUCTION POTENTIAL, THE STRONGER THE OXIDIZING AGENT WILL BE. This would say that fluorine gas is the best oxidizing agent in the list since F2 + 2e- 2F- has the most positive reduction potential of 2.87 volts THE MORE NEGATIVE THE STANDARD REDUCTION POTENTIAL (WHICH WOULD MAKE IT THE MORE POSITIVE OXIDATION POTENTIAL), THE STRONGER THE REDUCING AGENT WILL BE. This would say that Li metal is the best reducing agent in the list since. Li+ + e- Li has the most negative reduction potential of -3.05 volts (therefore most positive oxidation potential) ACTIVITY SERIES The above mentioned table can be used to compare the CHEMICAL REACTIVITY of one metal with another. The most ACTIVE metal on the chart is Li. It will replace EVERY metal above it. This works for every time for comparing the chemical activity of one metal with another. If the metal in question is BELOW the metal to be replaced – the first metal WILL replace the second. CALCULATING CELL POTENTIAL Remember E0cell = Ered + Eox EXAMPLE: Write the overall reaction and calculate the Eocell for the following galvanic cell: 2H+ + 2e- H2 Zn2+ + 2e- Zn Eored = 0.00 V Eored = -0.76V COMPLETE DESCRIPTION: As shown in the cell above, if a solid (metal) is not part of the half-reaction, an inert metal electrode(such as Platinum) is used. NONSTANDARD CELL POTENTIALS So far, we have only considered cells under standard conditions. This means a temperature of 298K, pressure of 1atm and ion concentrations of 1.0 M. Now consider NONSTANDARD conditions. What we will change to make the cell nonstandard is the concentration of ions – make them something other than 1.0 M. NERNST EQUATION E = Eo - (RT/nF)lnQ Combining constants at 25oC (298.15 K) gives the simpler form of the Nernst equation for an electrode reaction at this standard temperature: E = Eo - (.0592/n)log Q EXAMPLE Calculate the E for a galvanic cell based on the following half-reaction at 25oC. Cd2+ + 2e- Cd Eored = -0.40V 2+ Pb + 2e Pb Eored = -0.13V 2+ Where [Cd ] = 0.010 M and [Pb2+] = 0.100 M OTHER VERY IMPORTANT EQUATIONS FOR ELECTROCHEMICAL CELLS EQUATION TO DETERMINE THE GIBBS FREE ENERGY OF A STANDARD CELL G = nFE (*Remember- a galvanic cell (battery) at equilibrium is a “dead” battery! E = 0, Q = K) EQUATION RELATING EQUILIBRIUM CONSTANT ‘K’ TO ‘E o’ logK = (nEo) / 0.0592 ELECTROLYSIS ELECTROLYTIC CELL The above named cell will #1. Convert ELECTRICAL energy into CHEMICAL. #2. Be NONspontaneous going from LEFT to RIGHT which means (A) K < 1 (B) Eo < 0 (C) Go > 0 #3. Electrons will flow from the ANODE to the CATHODE. #4. Oxidation occurs at the ANODE. #5. Reduction occurs at the CATHODE. #6. The overall cell reaction is the sum of the ½ reaction at the anode + ½ reaction at the cathode. #7. AN OUTSIDE VOLTAGE SOURCE (BATTERY) IS NEEDED FOR THE CELL TO OPERATE. The process of electrolysis involves forcing a current through a cell to produce a chemical change for which the cell potential is negative. Electroplating: Electroplating consists of depositing a thin layer of a metal on another, either for protection or for the sake of appearance. For example: a brass or nickel object is coated with a layer of silver by making use of electrolysis of a silver solution, using the object to be coated as the cathode: In this example: the anode consist of pure silver, and the cathode is the object to be plated. The electrolyte is silver nitrate. The reactions are: At the anode: Ag Ag+ + eAt the cathode: Ag+ + eAg EXAMPLE PROBLEM: What mass of metallic silver will be deposited from silver nitrate solution when a current of 0.53 A is passed through it for 95 min? ELECTROLYSIS NET IONICS Electrolysis of salts can involve either molten salts or aqueous solutions of salt. EXAMPLE Electrolysis of molten copper (II) chloride Net Ionic: Reduction ½ Reaction: Oxidation ½ Reaction: Balanced Net Ionic: Eocell = __________ ____________ gas is produces at the ___________ EXAMPLE Electrolysis of CuCl2 (aq) REMEMBER: the reduction 1/2 reaction with the more positive E ored will occur and the oxidation ½ reaction with the more positive E0oxid (less negative Eored) will occur. (Also remember – the Group 1 and 2 metals will never be reduced, H2O will be instead, and no polyatomic ion will be oxidized – H2O will be instead) REDUCTION OXIDATION Balanced Net Ionic: A QUESTION COULD BE ASKED SUCH AS – WHAT GAS IS FORMED AND AT WHICH ELECTRODE? WHAT HAPPENS TO THE pH OF THE CELL AS IT OPERATES?