Energy in Thermal Processes
advertisement

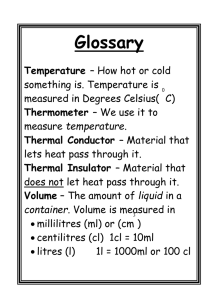
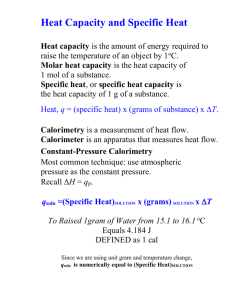
Energy in Thermal Processes Heat Calorimetry Phase Changes Heat and Internal Energy • _______ _______, U, is energy of atoms and molecules of a system – Includes KE of translation, rotation, vibration – Includes PE of chemical/electrical bonds • ______, Q, is transfer of energy between system and environment due to temperature difference ΔT Units of Heat • Historically defined in terms of __________ _________ of object – calorie (cal) – the energy necessary to raise the temperature of 1g of water from 14.5°C to 15.5°C – British thermal unit (BTU) – the energy necessary to raise the temperature of 1 lb of water from 63°F to 64°F • Heat (like work) is a transfer of energy and has SI units of ________ 1 cal 4.186 J Specific Heat • Every substance requires a ________ amount of energy per unit mass to change T by 1°C • Characterize the material by its _______ heat c • Units are J/(kg∙°C) • See Table 11.1, p. 355 Q 11.2 c mT Q mcT T T f Ti Calorimetry • Use containers that are good ________ (no energy leaves/enters the container) • Put warmer and cooler materials in container • Apply conservation of ______ Qcold Qhot In general Q k 0 Sign of ____ takes care of sign of Q Latent Heat and Phase Change • Sometimes energy transfer results in changes of ____ (melting/freezing = _____, boiling/condensing = _________) • _____________ does not change • Characterized by latent heat L • See Table 11.2, p. 360; units are J/kg Q mL 11.6 Use + if ______ energy, – sign if _______ energy Example: Ice to Steam Q mLv Q mcsteamT Q mcwater T Q mL f Q mciceT Fig. 11.3, p. 361