Density - Naval Aviation Museum Foundation
advertisement

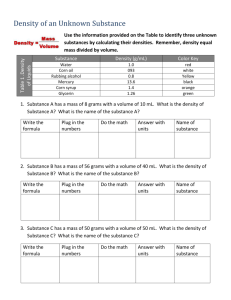
Answer Key Density Lab Density: mass per unit volume; unit—grams/milliliter *g/ml = g/cm3 Matter: anything that has mass and takes up space Mass: the amount of matter in an object; unit—grams Volume: the amount of space an object takes up; unit—milliliters Objectives: Mass an object Find the volume of an object Calculate density of an object Materials: Calculator Pencil Graduated cylinder (in ml) Water (with a drop or two of food color) Vegetable Oil Corn syrup Glycerin (with a drop of food color) Variety of small, solid objects (must fit in cylinder) ***I will use: rubber ball, wood block, plastic jack, plastic electrical cap, aluminum block, and lead block. Double-Pan or Triple-Beam Balance (in grams) Predict what you think will happen when each liquid is poured into a clear cylinder._______Answers will vary. _________________________________________ Predict what you think will happen to each object if dropped into the same cylinder with the liquids.________Answers will vary. _______________________________________ Complete the table as you follow the procedure. Object or Substance Mass, in g Volume, in ml (or cm3) Oil These have been completed Water for you.----------- -------------------Glycerin -------------------- -------------------Corn Syrup -------------------- -------------------Rubber ball 23.0 21.0 Plastic jack 3.9 2.0 Wood block 2.2 4.5 Plastic Electrical Cap 2.2 2.0 Al block 41.4 15.6 Pb block 175.7 15.6 Density in g/ml (or g/cm3) 0.93 1.00 1.26 1.38 1.10 1.95 0.49 1.10 2.65 11.26 ***You should add your own objects to the objects in the table to practice and extend this activity. Procedure: 1. Use the balance to mass each object. 2. To find the volume of each object, fill the cylinder to 50 ml (or a depth that will cover your objects) with water. Then, gently drop the object into the water. Measure the change in water level. This will be the volume of the object. Repeat with each object. 3. Now, use the following formula to find the density of each object. Density = mass/volume (or D = m/v) 4. Pour each liquid (oil, water, glycerin, corn syrup) with care into the graduated cylinder. Record what happens. The liquids will layer with the less dense above the more dense. 5. Now, drop each object carefully into the cylinder. Record what happens. The objects will float at the level above anything more dense. 6. Were your predictions correct? Explain. Answers will vary. Extension: If a golf ball has a mass of 45.7 g and a volume of 39.7 ml, calculate its density. D = m/v D = 45.7g/39.7ml D = 1.15 g/ml If the ball is dropped into the cylinder with the liquids (oil, water, glycerin, and corn syrup), at what level will the ball float? Explain. The ball should float between the water, with a density of 1.00 g/ml, and the glycerin, with a density of 1.26 g/ml. The golf ball is more dense than the water and less dense than the glycerin.