Density Worksheets
advertisement

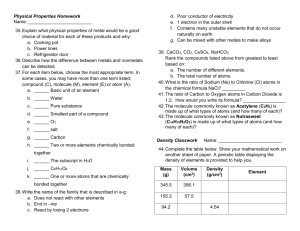
Name: ______________________________________________ Date: _____________________ Chapter 3 Density Calculations Calculate the following density problems. Don’t forget units! Report answers in correct number of significant figures. 1. A cork has a mass of 3.00 grams and a volume of 16.0 cm3. Calculate the density. 2. A block of aluminum occupies a space of 15.0 cm3 and weighs 40.5 g. What is its density? 3. A pencil has a density of 0.875 g/ cm3. It has a volume of 4.0 cm3. What is the mass? 4. A lead cylinder has a mass of 550.0 grams and a density of 2.70 g/ml. Calculate the volume of the lead cylinder. 5. A platinum bar measures 5.0 cm long, 4.2 cm wide, and 1.5 cm thick. It has a mass of 700.0 grams. a. Calculate the volume of the platinum bar. b. Calculate the density of the platinum bar. 6. A thin glass bottle holds 25 ml of liquid and has a mass of 19 grams. Calculate the density. 7. Measure the length, width, and height of each block using a ruler. Under each block you’ll find the mass, what is the density of each block? Write the density below the block. Block 1 – Mass = 98.6 g Density = _______________________________ Block 2 – Mass = 147.5 g Density = _______________________________ 8. The initial volume of water in a graduated cylinder is 2 ml. An irregularly shaped stone was lowered into this graduated cylinder and the height of the water rose to 7 ml. If the mass of the stone is 25 g, what is its density? Name: _____________________________ Date: _________________ Chapter 3 Density Density Problems HOMEWORK 1. Calculate the density for the following: a. 6.75 g solid with a volume of 5.35 cm3 b. a gas with a volume of 5.30 mL and a mass of 24.10 grams 2. An aluminum cylinder has a mass of 420 grams and a density of 2.70 g/ml. Calculate the volume of the aluminum cylinder. 3. What is the mass of ethyl alcohol that has a volume of 200.0 mL ? The density of ethyl alcohol is 0.789 g/mL. 4. A gold bar has a mass of 7,528 grams and a density of 19.32 g/cm3. Calculate the volume of the bar.