Calculations Involving Specific Heat and Latent Heat of
advertisement

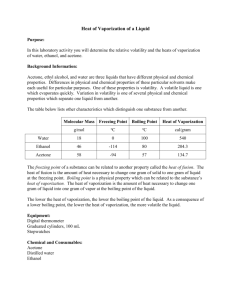
Name:_______________________________ Date:_______________ Period:______ 1) Temperature and Thermal Energy Key Ideas Temperature is a measure of the average kinetic energy of each particle within an object. Three temperature scales are Fahrenheit, Celsius, and Kelvin Fahrenheit scale – The temperature scale on which 32°F (water freezes) and 212°F (water boils). Celsius scale - The temperature scale on which 0°C (water freezes) and 100°C (water boils). Kelvin scale - The temperature scale on which zero is the temperature at which no more energy can be removed from matter. Also known as Absolute zero. Standard 4f There is no temperature lower than 0 Kelvin Thermal energy is the total energy of the particles that make up an object 2) The Nature of Heat Key Ideas Heat is a transfer of thermal energy from an object at a higher temperature to an object at a lower temperature. Heat is transferred by conduction, convection, and radiation. A conductor transfers heat well whereas an insulator does not. The amount of heat necessary to raise a given mass of a substance by a specific unit of temperature is called the specific heat. q = m(T)Cp where: q = heat energy m = mass T = temp. change Cp = specific heat 3) Quantifying energy: The traditional unit of energy is the calorie (cal), which is the amount of energy you need to add to 1 gram of water to heat it by 1 C. Food is measured in units of 1000 calories called kilocalories (kcal), which is more commonly known as the Calorie (Cal). The metric unit of energy is the joule (J). There are 4.184 J/cal. Because a joule isn’t very much energy, we usually measure energy in units of 1000 joules called kilojoules (kJ). Key Terms Specific heat –is the amount of heat per unit mass required to raise the temperature by one degree Celsius. The relationship between heat and temperature change is usually expressed in the form shown where c is the specific heat. The relationship does not apply if a phase change is encountered, because the heat added or removed during a phase change does not change the temperature. The specific heat of Water is Cp (H2O) = 1.00 cal/(gC) = 1.00 cal J 4.18 g C g C a. How many joules of energy must be absorbed to raise the temperature of 20 grams of water from 25°C to 30°C? Type 1. Heat Transferred (q) is the unknown: Ex. Aluminum has a specific heat of 0.902 J/g x oC. How much heat is lost when a piece of aluminum with a mass of 23.984 g cools from a temperature of 415.0 oC to a temperature of 22.0 o C? Step 1: First read the question and try to understand what they are asking you. Can you picture a piece of aluminum foil that is taken out of an oven. Imagine the aluminum losing heat to its surroundings until the temperature goes from 415.0 oC to 22.0 oC. Step 2: Write the original formula. q = m(T)Cp Step 3: List the known and unknown factors. Looking at the units in the word problem will help you determine which is which. q=? m = 23.984 g DT = (415.0 oC - 22.0 oC) = 393.0 oC (remember, they asked for the change in temperature) Cp = 0.902 J/g x oC Step 4. Substitute your values into the formula q=? m = 23.984 g DT = (415.0 oC - 22.0 oC) = 393.0 oC Cp = 0.902 J/g x oC q = m(T)Cp q = 23.984 g x 393.0 oC x 0.902 J/g x oC Step 5. Cross out units where possible, and solve for unknown. q = 23.984 g x 393.0 oC x 0.902 J/g x oC q = 8501.992224 J Step 6. Round to the correct number of significant digits and check to see that you answer makes sense. q = 8.50 x 103 J Our answer makes sense because joules (J) are acceptable units for q, and the value should be positive based on the wording of the question. Example An 50 gram sample of an unknown metal warms from 18° to 58° after absorbing 800 joules. What is the specific heat of the metal? When a substance is changing state, the temperature of the substance remains constant even though its thermal energy is changing. Change of state – The physical change of matter from one state to another. Melting – The change from a solid to the liquid form of matter. Melting point – The temperature at which a substance melts. Freezing – The change from the liquid to the solid form of matter. Freezing point – The temperature at which a substance freezes. Vaporization - The change from the liquid to the gaseous form of matter. Evaporation – Vaporization that occurs at the surface of a liquid. Boiling – Vaporization that occurs below the surface of a liquid. Boiling point – The temperature at which a liquid substance boils. Condensation – The change from the gaseous to the liquid form of matter. Freezing and Boiling Point Graph, Vapor Pressure and Boiling, Phase Diagram http://ess.lamar.edu/people/faculty/pittmanjg/teaching/the-oceans-online-fall-2006/assignments/atmospheric-and-oceanic-circulation/water-phase-changes-cool.jpg Energy During Change of States1 Heat of Fusion: The energy required to change a gram of a substance the solid to the liquid state without changing its temperature is commonly called it's "heat of fusion". This energy breaks down the solid bonds, but leaves a significant amount of energy associated with the intermolecular forces of the liquid state. The equation is Q = m Hfus where: Q = energy, m = mass and Hfus = heat of fusion The Hfus = heat of fusion for water is 80 cal/g = 334 J/g 1 http://hyperphysics.phy-astr.gsu.edu/Hbase/thermo/phase2.html#c1 from Heat of Vaporization: The energy required to change a gram of a liquid into the gaseous state at the boiling point is called the "heat of vaporization". This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the PV work applied). For an ideal gas, there is no longer any potential energy associated with intermolecular forces. So the internal energy is entirely in the molecular kinetic energy. The equation is Q = m Hvap where: Q = energy, m = mass and Hvap = heat of vaporization The Hvap = heat of vaporation for water is 540 cal/g = 2260 J/g A significant feature of the vaporization phase change of water is the large change in volume that accompanies it. A mole of water is 18 grams, and at STP that mole would occupy 22.4 liters if vaporized into a gas. If the change is from water to steam at 100°C, rather than 0°C, then by the ideal gas law that volume is increased by the ratio of the absolute temperatures, 373K/273K, to 30.6 liters. Comparing that to the volume of the liquid water, the volume expands by a factor of 30600/18 = 1700 when vaporized into steam at 100°C. This is a physical fact that firefighters know, because the 1700-fold increase in volume when water is sprayed on a fire or hot surface can be explosive and dangerous. One way to visualize this large volume change is to note the volume of 18 ml of water in a graduated cylinder as the volume occupied by Avogadro's number of water molecules in the liquid state. If converted into steam at 100°C this same mole of water molecules would fill a balloon 38.8 cm in diameter (15.3 inches). Since heat (energy that’s being transferred through thermal motion) is more important than work (for our purposes), how do we measure it? More definitions of use: System: Whatever we’re studying. o This can be practically anything. If we are studying what happens when we heat a pan on the stove, the pan will be the system we are studying. o If a system gets energy added to it, the amount of energy it has after the change is positive. Because of this, an endothermic process is any process in which energy is absorbed by the system we’re talking about. o If a system has energy taken away from it, the amount of energy it has after the change is negative. Because of this, an exothermic energy is any process in which energy is given off by the system we’re talking about. Surroundings: Everything outside the system. o When studying the pan above, the surroundings will primarily consist of the stove (because it’s putting energy into the pan), though it technically consists of everything but the pan. Universe: The system + the surroundings. o In a general sense, the universe consists of everything that exists anywhere. From a thermodynamic standpoint, the universe usually consists of whatever the system we’re referring to is as well as whatever is putting energy into or taking energy away from it. o Example: If we’re studying a space heater, the heater will be our system, the house will be our surroundings, and the universe will be the heater and the house together (we ignore the irrelevant rest of the world, since it doesn’t really play a part in anything). Science Help Online Worksheet 2-10a Heat Transfer Worksheet2 q = m(T)Cp Use the above formula to solve the problems below. Remember to list the known and unknowns. 1. How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oC to 18.0 oC? (remember the specific heat of water is 1.00 cal/g oC) 2. How much heat is lost when a 640 g piece of copper cools from 375 oC, to 26 oC? (The specific heat of copper is 0.38452 J/g oC) 3. The specific heat of iron is 0.4494 J/g oC. How much heat is transferred when a 24.7 kg iron ingot is cooled from 880 oC to 13 oC? 2 http://www.fordhamprep.org/gcurran/sho/sho/worksheets/worksht210a.htm Calculations Involving Specific Heat and Latent Heat of Phase Change3 Standard: Students know how to solve problems involving heat flow and temperature changes, using known values of specific heat and latent heat of phase change. 1. The specific heat of carbon (graphite) is 0.71 J/(g·°C). How much energy is given off as a 2 gram piece of graphite cools from 120°C to 20°C? 2. A sample of mercury has a mass of 500 grams. When it absorbs 280 joules, the temperature is found to have increased from 25°C to 29°C. What is the specific heat of mercury? 3. How much energy must be absorbed by 10 grams of steam in order to raise its temperature by 100°C? The specific heat of steam is 1.87J/(g·°C). 4. A 50 gram piece of iron warms from 0°C to 40°C while absorbing 900 joules. What is the specific heat of iron? 5. How much energy must be removed from 500 grams of water in order to cool it from 80°C to 40°C? The specific heat of water can be found on your periodic table. 3 http://www.sciencegeek.net/Chemistry/taters/Unit7Thermochemical.htm 6. When a 500 gram piece of brass cools from 100°C to 60°C, it is found to have given up 7600 joules of energy. What is the specific heat of the brass? 7. How much energy is required to melt 4 moles of ice at its melting point? Assume that the molar heat of fusion of ice is 6 kJ/mol. 8. How much energy is required to boil 4 moles of water at its boiling point? Assume that the molar heat of vaporization of water is 41 kJ/mol. 9. An engineer wants to be able to condense 10 moles of water vapor every minute. Assuming the steam is at 100°C, how much energy must be removed every minute? Assume that the molar heat of vaporization of water is 41 kJ/mol. 10. How much energy must be removed from 30 moles of liquid water at 0°C in order to convert it to ice? Assume that the molar heat of fusion of ice is 6 kJ/mol. 13. A sample of ice at 0°C melts after absorbing 300 kJ of heat. How many moles of H2O are contained in the sample? Assume that the molar heat of fusion of ice is 6 kJ/mol. 14. A sample of water at 100°C is converted to steam after absorbing 820 kJ of heat. How many moles of H2O are contained in the sample? Assume that the molar heat of vaporization of water is 41 kJ/mol. 15. How many joules of energy are needed to melt 54 grams of ice at its melting point? Assume that the molar heat of fusion of ice is 6 kJ/mol. 16. How many joules of energy are needed to boil 90 grams of water at its boiling point. Assume that the molar heat of vaporization of water is 41 kJ/mol. How is it that you can have both water and ice at 0 C and both water and steam at 100 C? A lot of energy goes into these phase transitions. Why doesn't it change the temperature? If you have steel and wood at 0 C, which feels colder? If you have steel and wood at 100 C, which feels hotter? Will hot water freeze into ice cubes faster than cold water in your freezer? If you have a cup of coffee which is too hot to drink, should you add cream to it immediately to cool it or let it stay black and sit for a while before adding cream? The object is to get it cool enough to drink in the shortest possible time. What is the difference between evaporation and boiling? If you heat a uniform metal plate with a hole in it, will the hole get larger or smaller? Heat flow is normally from a high temperature toward a low temperature region. How do you manage to cool your body on a July day when the temperature is 102 F (compared to 98.6 F normal body temperature)? Everyone knows that heat flows from a hot area to a cold area. How then does your refrigerator get it to flow from the inside of its freezing compartment to the warm outside, "uphill" for heat?