13.2 reading guide p..
advertisement

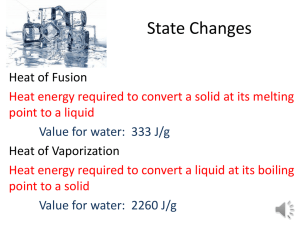
13.2 Energy and Water Page 427-430 Science 10 1. Why is it significant (important) that Earth’s surface is mainly covered in water? 2. Explain the difference in snow, ice and liquid water with regards to absorption of solar energy. 3. Define specific heat capacity. 4. Compare the specific heat capacity of water to other common materials., such as air and rocks such as granite and limestone. 5. Explain how a large specific heat capacity affects temperature changes in water. 6. Define heat of vaporization. 7. Compare the heat of vaporization of water to other liquids. 8. Explain how evaporation affects the temperature of water. 9. Define heat of fusion. 10. Compare the heat of fusion of water with other common liquids. 13.2 Energy and Water Page 427-430 Science 10 1. Why is it significant (important) that Earth’s surface is mainly covered in water? The energy interactions with water have a strong influence on the weather. 2. Explain the difference in snow, ice and liquid water with regards to absorption of solar energy. Water absorbs 93% of the energy that strikes it, while ice absorbs 50% and snow absorbs only 10%. 3. Define specific heat capacity. The amount of heat needed to raise the temperature of one gram of a substance by one degree Celsius 4. Compare the specific heat capacity of water to other common materials, such as air and rocks such as granite and limestone. Water has a specific heat capacity of about 4 times greater: Pure Water 4.18J/g°C Air 1.00J/g°C granite 0.79J/g°C limestone 0.92J/g°C 5. Explain how a large specific heat capacity affects temperature changes in water. Water changes its temperature slowly compared to other materials 6. Define heat of vaporization. The amount of heat needed to boil or evaporate 1 g of a liquid. 7. Compare the heat of vaporization of water to other liquids. Water has a very high heat of vaporization 8. Explain how evaporation affects the temperature of water. As water evaporates, large amounts of heat are removed from the rest of the liquid, causing it to stay cool. 9. Define heat of fusion. The amount of heat needed to melt 1 g of a solid at its melting point. 10. Compare the heat of fusion of water with other common liquids. Water also has a very high heat of fusion.