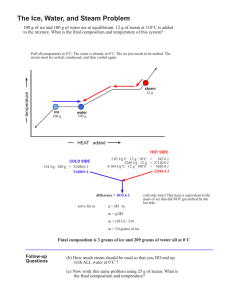
Heating Curve Practice Problems: AP Chemistry Worksheet
advertisement
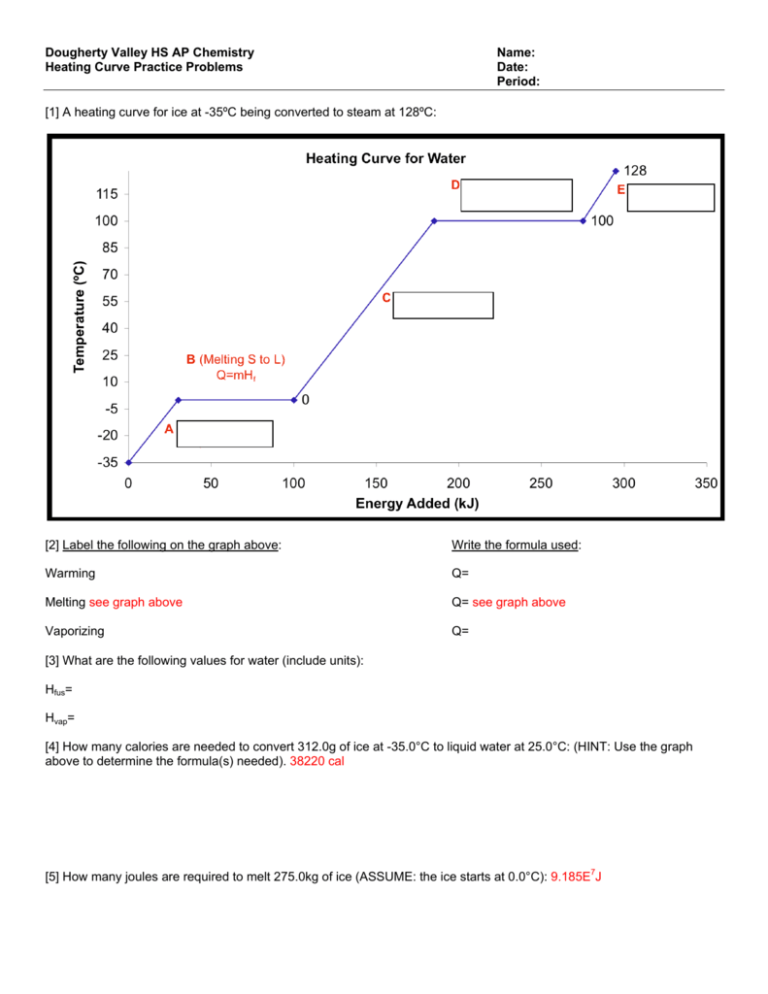
Dougherty Valley HS AP Chemistry Heating Curve Practice Problems Name: Date: Period: [1] A heating curve for ice at -35ºC being converted to steam at 128ºC: [2] Label the following on the graph above: Write the formula used: Warming Q= Melting see graph above Q= see graph above Vaporizing Q= [3] What are the following values for water (include units): Hfus= Hvap= [4] How many calories are needed to convert 312.0g of ice at -35.0°C to liquid water at 25.0°C: (HINT: Use the graph above to determine the formula(s) needed). 38220 cal [5] How many joules are required to melt 275.0kg of ice (ASSUME: the ice starts at 0.0°C): 9.185E7J 6. What mass of water (in kg) at 100.0°C could be completely vaporized with 2.70E3 cal of energy: 5.00E-3kg 7. How many joules (J) of energy are released when 6.80x103g of steam at 100.0°C are completely frozen to ice at 0.0°C: 2.05E7J 8. Convert the Hf value for water (80 cal/g) to units of J/mol: (HINT: You will need to use the molar mass of water) 6025 J/mol [9] How much energy (in J) is required to completely melt 205.0 mol of ice at 0.0°C: 1.235E6J Compound H2O K Hg Ag s (sol.) 𝑱 ( ) 𝒈∙𝑲 M.P. (C) Hfus 𝑱 ( ) s (liq.) 𝑱 ( ) 𝒈∙𝑲 B.P. (C) Hvap 𝑱 ( ) s (gas) 𝑱 ( ) 2.09 0.560 --0.217 0 62 -39 961 334 61.4 11 105 4.184 1.070 0.138 0318 100 760 357 2212 2260 2025 294 2355 1.97* 0.671 0.104 --- 𝒈 𝒈 *at 100C. The specific heat (s) of steam increases slightly with increasing temperature. For example, the Cp of steam at 200 C is 2.00. [10] How much heat is needed to raise the temperature of a 25.0 g sample of H2O from −25C to 125C? [11] How much heat is needed to raise the temperature of 85 g of potassium from 25C to 2,500C? 𝒈∙𝑲