Alkenes & Markownikoff's Rule
advertisement

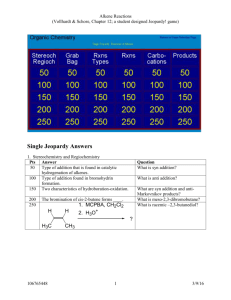
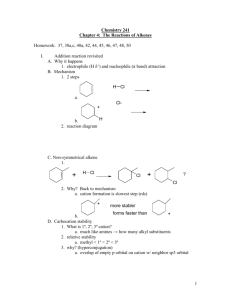
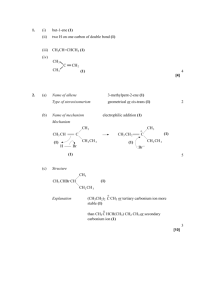
Alkenes & Markownikoff’s Rule Addition of Unsymmetrical Electrophiles to Unsymmetrical Alkenes Unsymmetrical electrophiles eg HBr compared to Br2 (Br – Br). Unsymmetrical Alkenes eg CH2 = CH – CH2 – CH3 compared to CH3 – CH = CH – CH3 When HBr attacks propene, there are two possible reaction pathways: - Route ‘A’ results in the formation of a secondary carbocation intermediate and is preferred to route ‘B’, which involves the formation of a primary carbocation intermediate. Secondary carbocations are more stable than primary because secondary ions have two alkyl groups bonded to the carbon atom carrying the positive charge. Alkyl groups have a +I inductive effect and push electrons from the C – C bond towards the + carbon atom, reducing the + charge on the carbon atom, so tending to spread the + charge throughout the molecule. Two alkyl groups directly bonded to the + carbon atom are more effective than one alkyl group even though it may be a larger alkyl group, as it is in the case of the primary carbocation. This “spreading out” of the positive charge stabilises the carbocation. Concentrated positive charge on an atom that is not particularly electropositive makes the ion very susceptible to nucleophilic attack or very unstable and unlikely to be formed. When 2 – methylpropene reacts with HBr, only one product results. Route ‘A’ involves the formation of a tertiary carbocation, which is far more stable than the primary carbocation involved in route ‘B’. Because there is a large difference in the relative stabilities of the carbocation intermediates, only one product is formed. Product mixtures result when carbocations of comparative stability are formed. 1 – methylcyclohexene forms only one product with HBr and that is 1–bromo–1–methylcyclohexane. Equal amounts of 2 – bromopentane and 3 – bromopentane are formed when HBr reacts with pent – 2 – ene. You should verify these last two statements for yourself. A rule that will help to remember which way an electrophile adds across a double bond is Markownikoff’s rule. It must be borne in mind that the rule is only an aid to memory and does not serve as an explanation of why the addition product is one molecule compared to another possible molecule. Markownikoff’s Rule When HX adds across a double bond of an alkene, the + H atom bonds onto the least substituted carbon atom (the one with the most H atoms bonded to it) whilst the X atom bonds to the most substituted carbon atom (the one with the least number of H atoms bonded to it). Practical Details The addition of a hydrogen halide to an alkene is carried out by bubbling the dry gaseous hydrogen halide directly into the alkene. A moderately polar solvent such as ethanoic acid can be used which will dissolve both the hydrogen halide and the alkene. The DRY gaseous hydrogen halide has to be used because the addition of an AQUEOUS solution of the hydrogen halide results in the formation of the alcohol as well as the desired haloalkane. This is because after very slow electrophilic attack by the H+ ion, very rapid nucleophilic attack on the resulting carbocation by water molecules follows. There are very many more water molecules present in aqueous solution than there are Br ions present. Reaction of Alkenes with Cold Concentrated Sulphuric Acid If the gaseous alkene is bubbled through cold, concentrated sulphuric acid, (liquid alkenes are added slowly, dropwise and mixed) alkyl hydrogensulphates are formed. The mechanism is exactly the same as in the case of attack by HBr. The carbocation intermediate formed by the initial electrophilic attack by + H is followed by nucleophilic attack. If this reaction is then followed by dilution of the product with water, or if dilute sulphuric acid is used in the first instance, propan–2–ol is formed. Either, the alkyl hydrogensulphate undergoes nucleophilic substitution by attack by water, the HSO4 nucleophile being replaced by H2O:, which then loses a proton. Or, in the case of reaction of the alkene with dilute H2SO4, water acts as a nucleophile after the initial protonation of the alkene. The reaction is a catalytic electrophilic hydration, as the mechanism shows. The mechanism shows the catalytic hydration of 2–methylpropene to produce 2–methylpropan–2–ol, which is of industrial importance. Remember, ethanol is made by the direct hydrolysis of ethene by mixing ethene with steam at 300 oC and 65 atmospheres pressure and passing the mixture over a phosphoric (V) acid on Celite catalyst. (See Page 2) Epoxyethane Epoxyethane is important in that it is used to make important derivatives, such as ethane–1,2–diol (antifreeze), non-ionic surfactants (detergents) and polyesters such as Terylene. Ethene is mixed with air and pressurised to about 10 atmospheres (1-2 Mpa). It is then passed over a catalyst of silver present as a finely divided layer on an alumina base at about 300°C. O Ag 2 CH2 = CH2 + O2 catalyst 2 CH2 – CH2 H = 210 kJ mol1 About 80% conversion takes place because some ethene is lost by oxidation to CO 2 and H2O. Great care must be taken during manufacture because epoxyethane is both flammable and explosive and the reaction must be carefully monitored because it is exothermic and the danger of explosion increases with increased temperature. An efficient system of heat exchangers is used and problems with static electricity and “hot spots” on the catalyst surface have to be avoided. It is also very toxic and affects the respiratory system and the brain. The gas is an excellent sterilising agent. The epoxyethane is a gas with a b.pt. of 10 oC and is purified by fractional distillation. Epoxyethane is very reactive towards nucleophiles because of its very strained ring system. With bond angles of 60 o, it doesn’t take much to open up the ring and bring about an exothermic reaction. Water 80% of all epoxyethane produced is converted into ethane – 1,2 – diol or antifreeze. Epoxyethane is mixed with water in a ratio of 1:10 in molar quantities, at 60 oC in the presence of sulphuric acid, which acts as a catalyst. The resulting solution is concentrated to 70% ethane–1,2– diol by evaporation and then further purified by fractional distillation. O CH2 – CH2 + H 2O HOCH2CH2OH H = 80 kJ mol1 First, the oxygen atom of the three-membered ring is protonated by the acid catalyst. The large excess of water is to try to ensure as high a yield as possible, but still only 90% conversion is achieved. The main side reaction or bi – product is HOCH2CH2OCH2CH2OH. This is formed by some of the ethane–1,2– diol product reacting with epoxyethane reactant. If the proportion of water is lowered in the hydration of epoxyethane, more complex polymeric products are formed in a stepwise series of reactions. These products are called “polyethylene glycols” and are used in the manufacture of plasticisers, polyurethanes and polyester resins, and which use they are put to depends on the value of ‘n’ in the equation. They are also used as solvents and lubricants. Ethane–1,2–diol, or glycol itself is used as an antifreeze in internal combustion engines. It has a low m.pt. of 12oC and a high b.pt. of 198 oC and it is completely miscible with water. A 60% mixture with water freezes 40oC. Ethane–1,2–diol is also used to make Terylene, a polymer fibre used in the clothing industry.